Chapter: Organic Chemistry: Alkyl halides
Alkyl halides: Factors affecting SN2 versus SN1 reactions
FACTORS AFFECTING SN2 VERSUS SN1
REACTIONS
Key Notes
SN1 versus SN2
The
nature of the nucleophile, the solvent, and the alkyl halide determine whether
nucleophilic substitution takes place by the SN1 or the SN2
mecha-nism. With polar aprotic solvents, primary alkyl halides react faster
than sec-ondary halides by the SN2 mechanism, whereas tertiary alkyl
halides hardly react at all. With polar protic solvents and nonbasic
nucleophiles, tertiary alkyl halides react faster than secondary alkyl halides
by the SN1 mechanism, and primary halides do not react. The reactivity of primary,
secondary, and tertiary alkyl halides is controlled by electronic and steric
factors.
Solvent
Polar,
aprotic solvents are used for SN2 reactions since they solvate
cations but not anions. As a result, nucleophiles are ‘naked’ and more
reactive. Protic solvent such
as water or
alcohol are used
in SN1 reactions
since they solvate and stabilize the intermediate carbocation. The
nucleophile is also solvated, but
this has no
effect on the reaction rate
since the rate is dependent on the concentration of the
alkyl halide.
Nucleophilicity
The rate
of the SN2 reaction increases with the nucleophilic strength of the incoming
nucleophile. The rate of the SN1 reaction is unaffected by the nature
of the nucleophile.
Leaving group
The
reaction rates of both the SN1 and the SN2 reaction is
increased if the leaving group is a stable ion and a weak base. Iodide is a
better leaving group than bromide and bromide is a better leaving group than
chloride. Alkyl fluorides do not undergo nucleophilic substitution.
Alkyl halides – SN2
Tertiary
alkyl groups are less likely to react by the SN2 mechanism than pri-
mary or secondary alkyl halides since the presence of three alkyl groups linked
to the reaction center lowers the electrophilicity of the alkyl halide by inductive effects.
Tertiary alkyl halides
have three bulky
alkyl groups attached to the
reaction center which act as steric shields and hinder the approach of
nucleophiles. Primary alkyl halides only have one alkyl group attached to this
center and so access is easier.
Alkyl halides – SN1
Formation
of a planar carbocation in the first stage of the SN1 mechanism is favored
for tertiary alkyl halides since it relieves the steric strain in the crowded
tetrahedral alkyl halide. The carbocation is also more accessible to an
incoming nucleophile. The formation of the carbocation is helped by electronic
factors involving the inductive and hyperconjugationeffects of the three
neighboring alkyl groups. Such inductive and hyperconjugation effects are
greater in carbocations formed from tertiary alkyl halides than from those
formed from primary or secondary alkyl halides.
Determining the mechanism
Measuring
how the reaction rate is affected by the concentration of the alkyl halide and
the nucleophile determines whether a nucleophilic substitution is SN2
or SN1. Measuring the optical activity of products from the
nucleo-philic substitution of asymmetric alkyl halides indicates the type of
mecha-nism involved. A pure enantiomeric product indicates an SN2
reaction. A partially or fully racemized product indicates an SN1
reaction.
SN1 versus SN2
There are two different mechanisms involved in
the nucleophilic substitution of alkyl
halides. When polar
aprotic solvents are
used, the SN2 mechanism
is preferred. Primary alkyl halides react more quickly than secondary
alkyl halides, with tertiary alkyl halides hardly reacting at all. Under protic
solvent conditions with nonbasic nucleophiles (e.g. dissolving the alkyl halide
in water or alcohol), the SN1 mechanism is preferred and the order
of reactivity is reversed. Tertiary alkyl halides are more reactive than
secondary alkyl halides, and primary alkyl halides do not react at all.
There are several factors which determine
whether substitution will be SN1 or SN2 and which also
control the rate at which these reactions take place. These include the nature
of the nucleophile and the type of solvent used. The reactivity of primary,
secondary, and tertiary alkyl halides is controlled by electronic and steric
factors.
Solvent
The SN2 reaction works best in polar
aprotic solvents (i.e. solvents with a high dipole moment, but with no O–H or
N–H groups). These include solvents such as acetonitrile (CH3CN) or dimethylformamide (DMF).
These solvents are
polar enough to dissolve the ionic reagents required for nucleophilic
substitution, but they do so by solvating the metal cation rather than the
anion. Anions are solvated by hydrogen bonding and since the solvent is
incapable of hydrogen bonding, the anions remain unsolvated. Such ‘naked’
anions retain their nucleophilicity and react more strongly with electrophiles.
Polar, protic solvents such as water or
alcohols can also dissolve ionic reagents but they solvate both the metal
cation and the anion. As a result, the anion is ‘caged’ in by solvent
molecules. This stabilizes the anion, makes it less nucleo- philic and makes it
less likely to react by the SN2 mechanism. As a result, the SN1
mechanism becomes more important.
The SN1 mechanism is particularly
favored when the polar protic solvent is also a nonbasic nucleophile.
Therefore, it is most likely to occur when an alkyl halide is dissolved in
water or alcohol. Protic solvents are bad for the SN2 mechanism since
they solvate the nucleophile, but they are good for the SN1
mechanism. This is because polar protic solvents can solvate and stabilize the
carbocation interme- diate. If the carbocation is stabilized, the transition
state leading to it will also be stabilized and this determines whether the SN1
reaction is favored or not. Protic solvents will also solvate the nucleophile
by hydrogen bonding, but unlike the SN2 reaction, this does not
affect the reaction rate since the rate of reaction is independent of the
nucleophile.
Nonpolar solvents are of no use in either the SN1
or the SN2 reaction since they cannot dissolve the ionic reagents
required for nucleophilic substitution.
Nucleophilicity
The relative nucleophilic strengths of incoming
nucleophiles will affect the rate of the SN2 reaction with stronger
nucleophiles reacting faster. A charged nucleophile is stronger than the
corresponding uncharged nucleophile (e.g. alkoxide ions are stronger
nucleophiles than alcohols). Nucleophilicity is also related to base strength
when the nucleophilic atom is the same (e.g. RO- > HO-
> RCO2- > ROH- > H2O). In
polar aprotic solvents, the order of nucleophilic strength for the halides is F-
> Cl- > Br- >I- .
Since the rate of the SN1 reaction
is independent of the incoming nucleophile, the nucleophilicity of the incoming
nucleophile is unimportant.
Leaving group
The nature of the leaving group is important to
both the SN1 and SN2 reactions – the better the leaving
group, the faster the reaction. In the transition states of both reactions, the
leaving group has gained a partial negative charge and the better that can be
stabilized, the more stable the transition state and the faster the reaction.
Therefore, the best leaving groups are the ones which form the most stable anions.
This is also related to basicity in the sense that the more stable the anion,
the weaker the base. Iodide and bromide ions are stable ions and weak bases,
and prove to be good leaving groups. The chloride ion is less stable, more
basic and a poorer leaving group. The fluoride ion is a very poor leaving group
and as a result alkyl fluorides do not undergo nucleophilic substitution. The
need for a stable leaving group explains why alcohols, ethers, and amines do
not undergo nucleophilic substitutions since they would involve the loss of a
strong base (e.g. RO or R2N- ).
Alkyl halides – SN2
There are two factors which affect the rate at
which alkyl halides undergo the SN2 reaction – electronic and
steric. In order to illustrate why different alkyl halides react at different
rates in the SN2 reaction, we shall compare a primary, secondary,
and tertiary alkyl halide (Fig. 1).
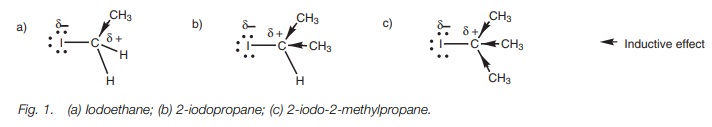
Alkyl groups have an inductive,
electron-donating effect which tends to lower the electrophilicity of the
neighboring carbon center. Lowering the electrophilic strength means that the
reaction center will be less reactive to nucleophiles. There-fore, tertiary
alkyl halides will be less likely to react with nucleophiles than primary alkyl
halides, since the inductive effect of three alkyl groups is greater than one
alkyl group.
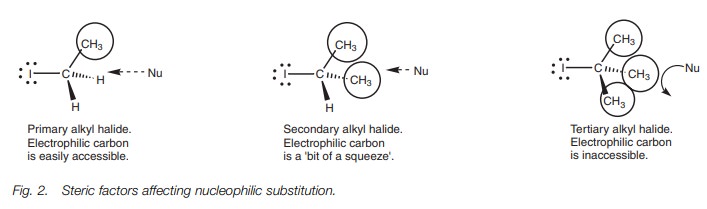
Steric factors also play a role in making the SN2 mechanism difficult for tertiary halides. An alkyl group is a bulky group compared to a hydrogen atom, and can therefore act like a shield against any incoming nucleophile (Fig. 2). A tertiary alkyl halide has three alkyl shields compared to the one alkyl shield of a primary alkyl halide. Therefore, a nucleophile is more likely to be deflected when it approaches a tertiary alkyl halide and fails to reach the electrophilic center.
Alkyl halides – SN1
Steric and electronic factors also play a role
in the rate of the SN1 reaction. Since the steric bulk of three
alkyl substituents makes it very difficult for a nucleophile to reach the
electrophilic carbon center of tertiary alkyl halides, these structures undergo
nucleophilic substitution by the SN1 mechanism instead. In this
mecha-nism, the steric problem is relieved because loss of the halide ion
creates a planar carbocation where the alkyl groups are much further apart and
where the carbon center is more accessible. Formation of the carbocation also
relieves steric strain between the substituents.
Electronic factors also help in the formation
of the carbocation since the positive charge can be stabilized by the inductive
and hyperconjugative effects of the three alkyl groups.
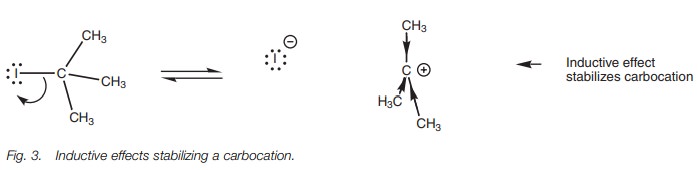
Both the inductive and hyperconjugation effects
are greater when there are three alkyl groups connected to the carbocation center
than when there are only one or two. Therefore, tertiary alkyl halides are far
more likely to produce a stable carbocation intermediate than primary or
secondary alkyl halides. It is important to realize that the reaction rate is
determined by how well the transition
state of the rate determining step is stabilized. In a situation like this
where a high energy intermediate is formed (i.e. the carbocation), the
transition state leading to it will be closer in character to the intermediate
than the starting material. Therefore, any factor which stabilizes the
intermediate carbocation also stabilizes the transition state and consequently
increases the reaction rate.
Determining the mechanism
It is generally fair to say that the
nucleophilic substitution of primary alkyl halides will take place via the SN2
mechanism, whereas nucleophilic substitution of ter-tiary alkyl halides will
take place by the SNl mechanism. In general, secondary alkyl halides
are more likely to react by the SN2 mechanism, but it is not
possible to predict this with certainty. The only way to find out for certain
is to try out the reaction and see whether the reaction rate depends on the
concentration of both reactants (SN2) or whether it depends on the
concentration of the alkyl halide alone (SNl).
If the alkyl halide is chiral the optical
rotation of the product could be measured to see whether it is a pure
enantiomer or not. If it is, the mechanism is SN2. If not, it is SN1.
Related Topics