Chapter: Organic Chemistry: Alkyl halides
Reactions of alkyl halides
REACTIONS OF ALKYL HALIDES
Key Notes
Nucleophilic substitution
Primary
and secondary alkyl halides can be converted to a large variety of different
functional groups such as alcohols, ethers, thiols, thioethers, esters, amines,
azides, alkyl iodides, and alkyl chlorides. Larger carbon skeletons can be
created by the reaction of alkyl halides with nitrile, acetylide, and enolate
ions. Substitution reactions of tertiary alkyl halides are more diffi-cult and
less likely to give good yields.
Eliminations
Elimination
reactions of alkyl halides allow the synthesis of alkenes and are best carried
out using a strong base in a protic solvent with heating. Pri-mary, secondary,
and tertiary alkyl halides can all be used. However, the use of a bulky base is
advisable for primary alkyl halides. The most substituted alkene is favored if
there are several possible alkene products.
Nucleophilic substitution
The nucleophilic substitution of alkyl halides
is one of the most powerful methods of obtaining a wide variety of different
functional groups. Therefore, it is possible to convert a variety of primary
and secondary alkyl halides to alcohols, ethers, thiols, thioethers,
esters, amines, and
azides (Fig. 1). Alkyl iodides and alkyl
chlorides can also be synthesized from other alkyl halides.
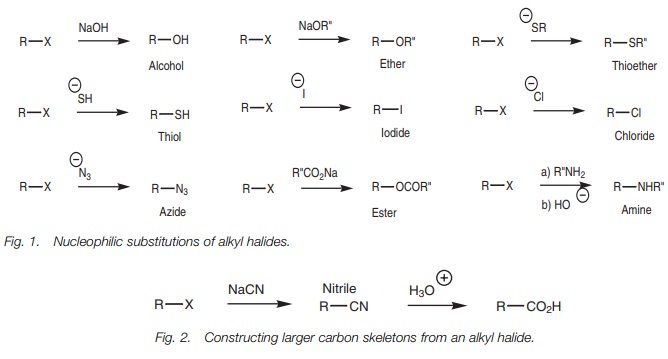
It is also possible to construct larger carbon skeletons using alkyl halides. A sim- ple example is the reaction of an alkyl halide with a cyanide ion (Fig. 2). This is an important reaction since the nitrile product can be hydrolyzed to give a carboxylic acid.
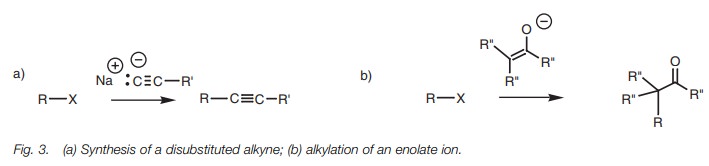
The reaction of an acetylide ion with a primary
alkyl halide allows the synthe-sis of disubstituted alkynes (Fig. 3a). The enolate ions of esters or
ketones can also be alkylated with alkyl halides to create larger carbon
skeletons. The most successful nucleophilic substitutions are with primary
alkyl halides. With secondary and tertiary alkyl halides, the elimination
reaction may compete, especially when the nucleophile is a strong base. The
substitution of ter-tiary alkyl halides is best done in a protic solvent with
weakly basic nucleophiles. However, yields may be poor.
Eliminations
Elimination reactions of alkyl halides (dehydrohalogenations) are a useful
method of synthesizing alkenes. For best results, a strong base (e.g. NaOEt)
should be used in a protic solvent (EtOH) with a secondary or tertiary alkyl
halide. The reaction proceeds by an E2 mechanism. Heating increases the chances
of elimination over substitution.
For primary alkyl halides, a strong, bulky base (e.g. NaOBut) should be used. The bulk hinders the possibility of the SN2 substitution and encourages elimina-tion by the E2 mechanism. The advantage of the E2 mechanism is that it is higher yielding than the E1 mechanism and is also stereospecific. The geometry of the product obtained is determined by the antiperiplanar geometry of the transition state. For example, the elimination in Fig. 4 gives the E-isomer and none of the Z-isomer.
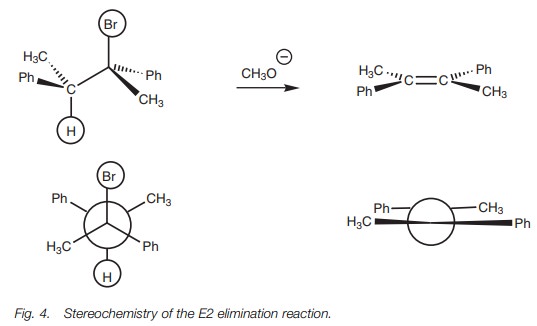
If the elimination occurs by the E1 mechanism,
the reaction is more likely to compete with the SN1 reaction and a
mixture of substitution and elimination products is likely.
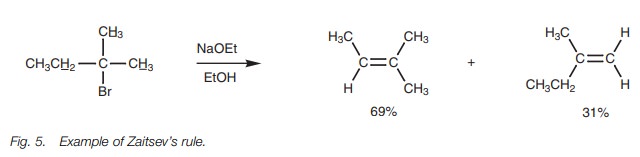
The E2 elimination requires the presence of a β-proton. If there are several options available, a mixture of
alkenes will be obtained, but the favored alkene will be the most substituted
(and most stable) one (Zaitsev’s rule;
Fig. 5).
The transition state for the reaction resembles
the product more than the reac-tant and so the factors which stabilize the
product also stabilize the transition state and make that particular route more
likely. However, the opposite preference is found when potassium tert-butoxide is used as base, and the
less substituted alkene is favored.
Related Topics