Chapter: Organic Chemistry: Alkyl halides
Alkyl halides: Elimination versus substitution
ELIMINATION VERSUS SUBSTITUTION
Key Notes
Introduction
The
ratio of substitution and elimination products formed from alkyl halides
depends on the reaction conditions as well as the nature of the nucleophile and
the alkyl halide.
Primary alkyl halides
Primary
alkyl halides will usually undergo SN2 substitution reactions in
preference to E2 elimination reactions. However, the E2 elimination reaction is
favored if a strong bulky base is used with heating.
Secondary alkyl halides
Substitution
by the SN2 mechanism is favored over the E2 elimination if the
nucleophile is a weak base and the solvent is polar and aprotic. E2
elimina-tion is favored over the SN2 reaction if a strong base is
used in a protic solvent. Elimination is further favored by heating. SN1
and E1 reactions may be possible when dissolving secondary alkyl halides in
protic solvents.
Tertiary alkyl halides
E2 elimination
occurs virtually exclusively
if a tertiary
alkyl halide is treated with a strong base in a protic
solvent. Heating a tertiary alkyl halide in a protic solvent is likely to
produce a mixture of SN1 substitution and E1 elimination products,
with the former being favored.
Introduction
Alkyl halides can undergo both elimination and
substitution reactions and it is not unusual to find both substitution and
elimination products present. The ratio of the products will depend on the
reaction conditions, the nature of thenucleophile and the nature of the alkyl
halide.
Primary alkyl halides
Primary alkyl halides undergo the SN2
reaction with a large range of nucleophiles (e.g. RS- , I- , CN- , NH3, or Br- ) in polar aprotic solvents such as
hexamethyl- phosphoramide (HMPA; [(CH3)2N]3PO).
However, there is always the possibility of some E2 elimination occurring as
well. Nevertheless, substitution is usually favored over elimination, even when
using strong bases such as HO- or EtO-. If E2 elimination
of a primary halide is desired, it is best to use a strong bulky base such as tert-butoxide [(CH3)3C–O- ]. With a bulky base, the elimination product
isfavored over the substitution product since the bulky base experiences more
steric hindrance in its approach to the electrophilic carbon than it does to
the acidic β-proton.
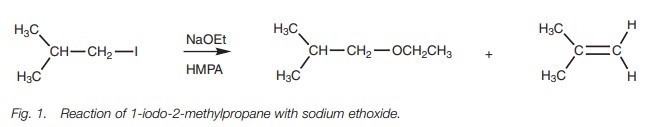
Thus, treatment of a primary halide (Fig. 1) with an ethoxide ion is likely to give a mixture of an ether arising from SN2 substitution along with an alkene arising from E2 elimination, with the ether being favored. By using sodium tert-butoxide instead, the preferences would be reversed.
Increasing the temperature of the reaction
shifts the balance from the SN2 reaction to the elimination
reaction. This is because the elimination reaction has a higher activation
energy due to more bonds being broken. The SN1 and E1 reactions do
not occur for primary alkyl halides.
Secondary alkyl halides
Secondary alkyl halides can undergo both SN2
and E2 reactions to give a mixture of products. However, the substitution product
predominates if a polar aprotic solvent is used and the nucleophile is a weak
base. Elimination will predominate if a strong base is used as the nucleophile
in a polar, protic solvent. In this case, bulky bases are not so crucial and
the use of ethoxide in ethanol will give more elimination product than
substitution product. Increasing the temperature of the reaction favors E2
elimination over SN2 substitution as explained above.
If weakly basic or nonbasic nucleophiles are
used in protic solvents, elimination and substitution may occur by the SN1
and E1 mechanisms to give mixtures.
Tertiary alkyl halides
Tertiary alkyl halides are essentially unreactive to strong nucleophiles in polar, aprotic solvents – the conditions for the SN2 reaction. Tertiary alkyl halides can undergo E2 reactions when treated with a strong base in a protic solvent and will do so in good yield since the SN2 reaction is so highly disfavored. Under nonbasic conditions in a protic solvent, E1 elimination and SN1 substitution both take place.
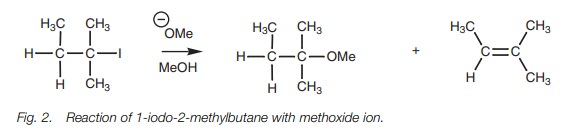
A tertiary alkyl halide treated with sodium
methoxide could give an ether or an alkene (Fig.
2). A protic solvent is used here and this favors both the SN1
and E1 mechanisms. However, a strong base is also being used and this favors
the E2 mechanism. Therefore, the alkene would be expected to be the major
product with only a very small amount of substitution product arising from the
SN2 reaction. Heating the same alkyl halide in methanol alone means
that the reaction is being carried out in a protic solvent with a nonbasic
nucleophile (MeOH). These condi-tions would result in a mixture of substitution
and elimination products arising from the SN1 and E1 mechanisms. The
substitution product would be favored over the elimination product.
Related Topics