Chapter: Basic & Clinical Pharmacology : Heavy Metal Intoxication & Chelators
Pharmacology of Chelators
PHARMACOLOGY OF CHELATORS
Chelating
agents are drugs used to prevent or reverse the toxic effects of a heavy metal
on an enzyme or other cellular target, or to accelerate the elimination of the
metal from the body. By form-ing a complex with the heavy metal, the chelating
agent renders the metal unavailable for toxic interactions with functional
groups of enzymes or other proteins, coenzymes, cellular nucleophiles, and
membranes. Chelating agents contain one or more coordinat-ing atoms, usually
oxygen, sulfur, or nitrogen, which donate a pair of electrons to a cationic
metal ion to form one or more coordi-nate-covalent bonds. Depending on the
number of metal-ligand bonds, the complex may be referred to as mono-, bi-, or
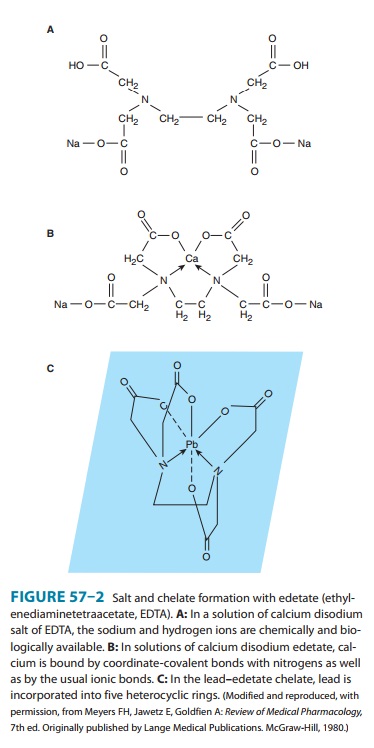
polyden-tate. Figure 57–2 depicts the hexadentate chelate formed by
inter-action of edetate (ethylenediaminetetraacetate) with a metal atom, such
as lead.
In some cases, the
metal-mobilizing effect of a therapeutic chelating agent may not only enhance
that metal’s excretion—a desired effect—but may also redistribute some of the
metal to other vital organs. This has been demonstrated for dimercaprol, which
redistributes mercury and arsenic to the brain while also enhancing urinary
mercury and arsenic excretion. Although several chelating agents have the
capacity to mobilize cadmium, their tendency to redistribute cadmium to the
kidney and increase nephrotoxicity has negated their therapeutic value in
cadmium intoxication.
In addition to
removing the target metal that is exerting toxic effects on the body, some
chelating agents may enhance excretion of essential cations, such as zinc in
the case of calcium EDTA and diethylenetriaminepentaacetic acid (DTPA), and
zinc and copper in the case of succimer. No clinical significance of this
effect has been demonstrated, although some animal data suggest the possibility
of adverse developmental impact. If prolonged chelation during the prenatal
period or early childhood period is necessary, judicious supplementation of the
diet with zinc might be considered.
The
longer the half-life of a metal in a particular organ, the less effectively it
will be removed by chelation. For example, in the case of lead chelation with
calcium EDTA or succimer, or of pluto-nium chelation with DTPA, the metal is
more effectively removed from soft tissues than from bone, where incorporation
into bone matrix results in prolonged retention.
In most cases, the capacity of chelating agents to prevent or reduce the adverse effects of toxic metals appears to be greatest when such agents are administered very soon after an acute metal exposure. Use of chelating agents days to weeks after an acute metal exposure ends—or their use in the treatment of chronic metal intoxication—may still be associated with increased metal excretion. However, at that point, the capacity of such enhanced excretion to mitigate the pathologic effect of the metal exposure may be reduced.
Related Topics