Chapter: Basic & Clinical Pharmacology : Heavy Metal Intoxication & Chelators
Edetate Calcium Disodium (Ethylenediaminetetraacetic Acid, EDTA)
EDETATE CALCIUM DISODIUM
(ETHYLENEDIAMINETETRAACETIC ACID, EDTA)
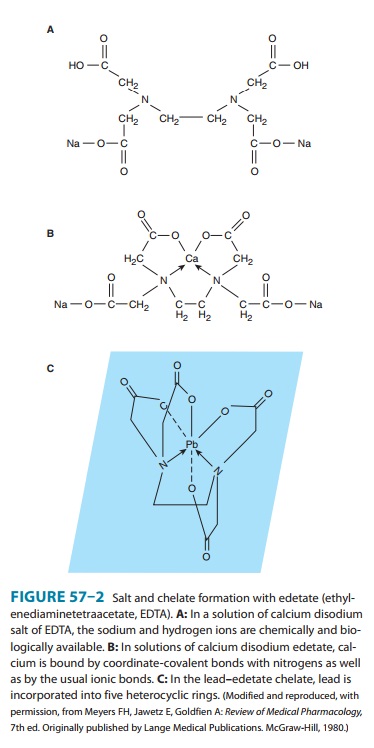
Ethylenediaminetetraacetic
acid (Figure 57–2) is an efficient che-lator of many divalent and trivalent
metals in vitro. To prevent potentially life-threatening depletion of calcium,
the drug should be administered only as the calcium disodium salt.EDTA penetrates cell
membranes relatively poorly and there-fore chelates extracellular metal ions
much more effectively than intracellular ions.
The highly polar ionic
character of EDTA limits its oral absorption. Moreover, oral administration may
increase lead absorption from the gut. Consequently, EDTA should be
admin-istered by intravenous infusion. In patients with normal renal function,
EDTA is rapidly excreted by glomerular filtration, with 50% of an injected dose
appearing in the urine within 1 hour. EDTA mobilizes lead from soft tissues,
causing a marked increase in urinary lead excretion and a corresponding decline
in blood lead concentration. In patients with renal insufficiency, excretion of
the drug—and its metal-mobilizing effects—may be delayed.
Indications & Toxicity
Edetate calcium
disodium is indicated chiefly for the chelation of lead, but it may also have
usefulness in poisoning by zinc, manga-nese, and certain heavy radionuclides.
In spite of repeated claims in the alternative medicine literature, EDTA has no
demonstrated use-fulness in the treatment of atherosclerotic cardiovascular
disease.
Because the drug and
the mobilized metals are excreted via the urine, the drug is relatively
contraindicated in anuric patients. In such instances, the use of low doses of
EDTA in combination with high-flux hemodialysis or hemofiltration has been
described. Nephrotoxicity from EDTA has been reported, but in most cases can be
prevented by maintenance of adequate urine flow, avoid-ance of excessive doses,
and limitation of a treatment course to5 or fewer consecutive days. EDTA may
result in temporary zinc depletion that is of uncertain clinical significance.
Analogs of EDTA, the calcium and zinc disodium salts of DTPA, pentetate, have
been used for removal (“decorporation”) of certain tran-suranic, rare earth,
and transition metal radioisotopes, and in 2004 were approved by the FDA for
treatment of contamination with plutonium, americium, and curium.
Related Topics