Chapter: Medical Surgical Nursing: Management of Patients With Upper or Lower Urinary Tract Dysfunction
Peritoneal Dialysis
PERITONEAL
DIALYSIS
The goals of peritoneal dialysis are to remove toxic substances and metabolic wastes and to re-establish normal fluid and electrolyte balance. Peritoneal dialysis may be the treatment of choice for pa-tients with renal failure who are unable or unwilling to undergo hemodialysis or renal transplantation. Patients who are suscepti-ble to the rapid fluid, electrolyte, and metabolic changes that occur during hemodialysis experience fewer of these problems with the slower rate of peritoneal dialysis. Therefore, patients with diabetes or cardiovascular disease, many older patients, and those who may be at risk for adverse effects of systemic heparin are likely candidates for peritoneal dialysis. Additionally, severe hypertension, heart failure, and pulmonary edema not responsive to usual treatment regimens have been successfully treated with peritoneal dialysis.
Peritoneal
dialysis can be performed using several different ap-proaches: acute,
intermittent peritoneal dialysis; continuous
am-bulatory peritoneal dialysis (CAPD); and continuous cyclic peritoneal dialysis (CCPD). These three methods
are discussed later. As with other forms of treatment, the deci-sion to begin
peritoneal dialysis is made by the patient and fam-ily in consultation with the
physician.
Although
specific patient populations do benefit from peri-toneal dialysis, it is not as
efficient as hemodialysis (Lindsay & Kortas, 2001). Because cardiovascular
disease is the cause of death in half of all patients with ESRD, the adequacy
of dialysis must be defined, in part, by its potential to reduce cardiovascular
dis-ease. Blood pressure, volume, left ventricular hypertrophy, and
dyslipidemias are the major causes of morbidity and mortality in patients
undergoing peritoneal dialysis (Chatoth, Golper & Gokal, 1999).
Underlying Principles
In
peritoneal dialysis, the peritoneum, a serous membrane that covers the
abdominal organs and lines the abdominal wall, serves as the semipermeable
membrane. The surface of the peritoneum constitutes a body surface area of about
22,000 cm2. Sterile dialysate
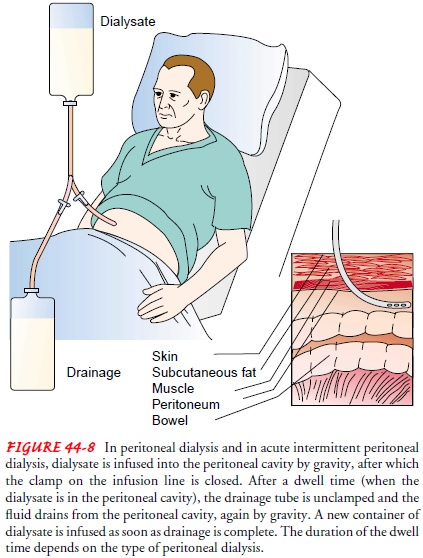
fluid is introduced into the peritoneal cavity through an abdominal catheter at
intervals (Fig. 44-8). Urea and creatinine, metabolic end products normally
excreted by the kidneys, are cleared from the blood by diffusion and ossmosis
as waste products move from an area of higher concentration (the peritoneal
blood supply) to an area of lower concentration (the peritoneal cavity) across
a semipermeable membrane (the peritoneal membrane). Urea is cleared at a rate
of 15 to 20 mL/min, whereas creatinine is removed at a slower rate. It usually
takes 36 to 48 hours to achieve with peritoneal dialysis what hemodialysis
accomplishes in 6 to 8 hours. Ultrafiltration (water removal) occurs in
peritoneal dialysis through an osmotic gradient created by using a dialysate
fluid with a higher glucose concentration.
Procedure
The patient undergoing peritoneal dialysis may be acutely ill, thus requiring short-term treatment to correct severe distur-bances in fluid and electrolyte status, or may have chronic renal failure and need to receive ongoing treatments.
PREPARING THE PATIENT
The
nurse’s preparation of the patient and family for peritoneal dialysis depends
on the patient’s physical and psychological sta-tus, level of alertness,
previous experience with dialysis, and un-derstanding of and familiarity with
the procedure.
The
nurse explains the procedure to the patient and obtains signed consent for it.
Baseline vital signs, weight, and serum elec-trolyte levels are recorded. The
patient is encouraged to empty the bladder and bowel to reduce the risk of
puncturing internal organs. The nurse also assesses the patient’s anxiety about
the pro-cedure and provides support and instruction. Broad-spectrum antibiotic
agents may be administered to prevent infection. If the peritoneal catheter is
to be inserted in the operating room, this is explained to the patient and
family.
PREPARING THE EQUIPMENT
In
addition to assembling the equipment for peritoneal dialysis, the nurse
consults with the physician to determine the concen-tration of dialysate to be
used and the medications to be added to it. Heparin may be added to prevent
blood clotting and resultant occlusion of the peritoneal catheter. Potassium
chloride may be prescribed to prevent hypokalemia. Antibiotics may be added to
treat peritonitis. Insulin may be added for diabetic patients; a
larger-than-normal dose may be needed, however, because about 10% of the
insulin binds to the dialysate container. All medica-tions are added
immediately before the solution is instilled. Asep-tic technique is crucial.
Before
medications are added, the dialysate is warmed to body temperature to prevent
patient discomfort and abdominal pain and to dilate the vessels of the
peritoneum to increase urea clearance. Solutions that are too cold cause pain
and vasoconstriction and reduce clearance. Solutions that are too hot burn the
peritoneum. Dry heating is recommended (heating cabinet, incubator, or heat-ing
pad). Microwave heating of the fluid is not recommended be-cause of the danger
of burning the peritoneum.
Immediately
before initiating dialysis, the nurse assembles the administration set and
tubing. The tubing is filled with the pre-pared dialysate to reduce the amount
of air entering the catheter and peritoneal cavity, which could increase
abdominal discom-fort and interfere with instillation and drainage of the
fluid.
INSERTING THE CATHETER
Ideally,
the peritoneal catheter is inserted in the operating room to maintain surgical
asepsis and minimize the risk of contamina-tion. In some circumstances,
however, the physician inserts the catheter at the bedside under strict
asepsis.
A
rigid stylet catheter is inserted for acute peritoneal dialysis use only.
Before the procedure, the skin is prepared with a local antiseptic to reduce
skin bacteria and the risk of contamination and infection. The physician
anesthetizes the site with a local anesthetic agent before making a small
incision or stab wound in the lower abdomen, 3 to 5 cm below the umbilicus.
Because this area is relatively free from large blood vessels, little bleeding
oc-curs. A trocar is used to puncture the peritoneum as the patient tightens
the abdominal muscles by raising the head. The catheter is threaded through the
trocar and positioned. Previously pre-pared dialysate is infused into the
peritoneal cavity, pushing the omentum (peritoneal lining extending from the
abdominal or-gans) away from the catheter. The physician may then secure the
catheter with a purse-string suture and apply antibacterial oint-ment and a
sterile dressing over the site.
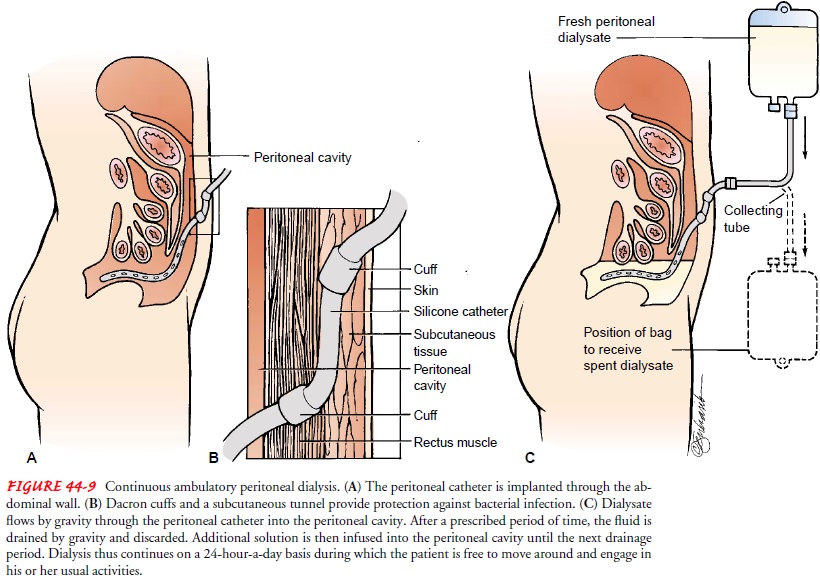
Catheters
for long-term use (Tenckhoff, Swan, Cruz) are usu-ally made of silicone and are
radiopaque to permit visualization on x-ray. These catheters have three
sections: (1) an intraperi-toneal section, with numerous openings and an open
tip to let dialysate flow freely; (2) a subcutaneous section that passes from
the peritoneal membrane and tunnels through muscle and sub-cutaneous fat to the
skin; and (3) an external section for connec-tion to the dialysate system. Most
of these catheters have two cuffs, which are made of Dacron polyester. The
cuffs stabilize the catheter, limit movement, prevent leaks, and provide a
barrier against microorganisms. One cuff is placed just distal to the
peri-toneum, and the other cuff is placed subcutaneously. The subcu-taneous
tunnel (5 to 10 cm long) further protects against bacterial infection (Fig.
44-9).
PERFORMING THE EXCHANGE
Peritoneal
dialysis involves a series of exchanges or cycles. An ex-change is defined as
the infusion, dwell, and drainage of the dialysate. This cycle is repeated
throughout the course of the dial-ysis. The dialysate is infused by gravity
into the peritoneal cavity. A period of about 5 to 10 minutes is usually
required to infuse 2 L of fluid. The prescribed dwell, or equilibration, time
allows dif-fusion and osmosis to occur. Diffusion of small molecules, such as
urea and creatinine, peaks in the first 5 to 10 minutes of the dwell time. At
the end of the dwell time, the drainage portion of the exchange begins. The
tube is unclamped and the solution drains from the peritoneal cavity by gravity
through a closed sys-tem. Drainage is usually completed in 10 to 30 minutes.
The drainage fluid is normally colorless or straw-colored and should not be
cloudy. Bloody drainage may be seen in the first few ex-changes after insertion
of a new catheter but should not occur after that time. The entire exchange
(infusion, dwell time, and drainage) takes 1 to 4 hours, depending on the
prescribed dwell time. The number of cycles or exchanges and their frequency
are prescribed based on the patient’s physical status and acuity of illness.
The removal of excess water during peritoneal dialysis is achieved by using a hypertonic dialysate with a high dextrose con-centration that creates an osmotic gradient. Dextrose solutions of 1.5%, 2.5%, and 4.25% are available in several volumes, from 500 mL to 3,000 mL, allowing the dialysate selection to fit the patient’s tolerance, size, and physiologic needs. The higher the dex-trose concentration, the greater the osmotic gradient and the more water removed. Selection of the appropriate solution is based on the patient’s fluid status.
Complications of Peritoneal Dialysis
Peritoneal
dialysis is not without complications. Most are minor, but several, if
unattended, can have serious consequences.
PERITONITIS
Peritonitis (inflammation of the peritoneum) is the most
com-mon and most serious complication of peritoneal dialysis. The or-ganism
responsible for peritoneal dialysis-related peritonitis is an important factor
in clinical outcome and the basis of treatment guidelines. There has been a
significant decrease in the rate of cases of peritonitis, from 1.37
episodes/patient-year in 1991 to 0.55 episodes/patient-year in 1998. Staphylococcus aureus and Staphylococcus epidermidis remain the
most common Gram-positiveorganisms responsible for peritonitis, although the
rates of each have decreased. Pseudomonas
aeruginosa, E. coli, and Klebsiella
species are the most common causes of Gram-negative peritonitis. Resistance to
antibacterial agents (ie, ciprofloxacin, methicillin) used in their treatment
increased dramatically from 1991 to 1998 (Zelenitsky et al., 2000).
Peritonitis
is characterized by cloudy dialysate drainage, dif-fuse abdominal pain, and
rebound tenderness. Hypotension and other signs of shock may occur if S. aureus is the responsible or-ganism.
The patient with peritonitis may be treated as an inpa-tient or outpatient
(most common), depending on the severity of the infection and the patient’s
clinical status. Initially, one to three rapid exchanges with a 1.5% dextrose
solution without added medications are completed to wash out mediators of
in-flammation and to reduce abdominal pain. Drainage fluid is ex-amined for
cell count, and Gram’s stain and culture are used to identify the organism and
guide treatment. Antibiotic agents (aminoglycosides or cephalosporins) are
usually added to subse-quent exchanges until the Gram’s stain or culture
results are avail-able for appropriate antibiotic determination.
Intraperitoneal administration of antibiotics is as effective as intravenous
admin-istration. Antibiotic therapy continues for 10 to 14 days. Careful
calculation of the antibiotic dosage helps prevent nephrotoxicity and further
compromise of renal function.
Heparin
(500 to 1,000 U/L) may be added to the dialysate to prevent fibrin clot
formation; oral administration of low-dose warfarin (Coumadin) is also
effective in decreasing coagulation factors and preventing thrombosis without
increasing the risk of bleeding (Kim, Lee, Park et al., 2001).
Peritonitis
that is unresolved after 4 days of appropriate ther-apy necessitates catheter
removal. The patient is maintained on hemodialysis for about 1 month before a
new catheter is inserted. In patients with fungal peritonitis, the peritoneal
catheter must be removed if there is no response to therapy in 4 to 7 days.
Tun-nel infections and fecal peritonitis also necessitate catheter re-moval.
Systemic antibiotics should continue for 5 to 7 days after catheter removal.
Regardless
of which organism causes peritonitis, the patient with peritonitis loses large
amounts of protein through the peri-toneum. Acute malnutrition and delayed
healing may result. Therefore, attention must be given to detecting and
promptly treating the infections.
LEAKAGE
Leakage
of dialysate through the catheter site may occur imme-diately after the
catheter is inserted. Usually, the leak stops spon-taneously if dialysis is
withheld for several days to give the incision and exit site time to heal.
During this time, it is important to reduce factors that might delay healing,
such as undue abdominal muscle activity and straining during bowel movement.
Leakage through the exit site or into the abdominal wall can occur for months
or years after catheter placement. In many cases, leakage can be avoided by
using small volumes (100 to 200 mL) of dialysate, gradually increasing the volume
up to 2,000 mL.
BLEEDING
A
bloody effluent (drainage) may be observed occasionally, espe-cially in young,
menstruating women. (The hypertonic fluid pulls blood from the uterus, through
the opening in the fallopian tubes, and into the peritoneal cavity.) Bleeding
is common during the first few exchanges after a new catheter insertion because
some blood exists in the abdominal cavity from the procedure. In many cases, no
cause can be found for the bleeding, although catheter displacement from the
pelvis has occasionally been associated with bleeding. Some patients have had
bloody effluent after an enema or from minor trauma. Invariably, bleeding stops
in 1 to 2 days and requires no specific intervention. More frequent exchanges
during this time may be necessary to prevent blood clots from obstructing the
catheter.
LONG-TERM COMPLICATIONS
Hypertriglyceridemia
is common in patients undergoing long-term peritoneal dialysis, suggesting that
this therapy may accelerate ath-erogenesis. Despite this, the use of
cardioprotective medications is relatively low, and many patients have
suboptimal blood pres-sure control. Given the high burden of disease in these
patients, beta-blockers and angiotensin-converting enzyme inhibitors should be
used to control hypertension or protect the heart; the use of aspirin and
statins should be considered. In general, health care providers need to be
better educated in this area of dialysis man-agement (Tonelli et al., 2001).
Other
complications that may occur with long-term peri-toneal dialysis include
abdominal hernias (incisional, inguinal, diaphragmatic, and umbilical),
probably resulting from continu-ously increased intra-abdominal pressure. The
persistently ele-vated intra-abdominal pressure also aggravates symptoms of
hiatal hernia and hemorrhoids. Low back pain and anorexia from fluid in the
abdomen and a constant sweet taste related to glucose absorption may also
occur.
Mechanical
problems occasionally occur and may interfere with instillation or drainage of
the dialysate. Formation of clots in the peritoneal catheter and constipation
are factors that may contribute to these problems.
Acute Intermittent Peritoneal Dialysis
Indications
for acute intermittent peritoneal dialysis, a variation of peritoneal dialysis,
include uremic signs and symptoms (nau-sea, vomiting, fatigue, altered mental
status), fluid overload, aci-dosis, and hyperkalemia. Although peritoneal
dialysis is not as efficient as hemodialysis in removing solute and fluid, it
permits a more gradual change in the patient’s fluid volume status and in waste
product removal. Therefore, it may be the treatment of choice for the
hemodynamically unstable patient. It can be car-ried out manually (the nurse
warms, spikes, and hangs each con-tainer of dialysate) or by a cycler machine.
Exchange times range from 30 minutes to 2 hours. A common routine is hourly
ex-changes consisting of a 10-minute infusion, a 30-minute dwell time, and a
20-minute drain time.
Maintaining the peritoneal dialysis cycle is
a nursing respon-sibility. Strict aseptic technique is maintained when changing
so-lution containers and emptying drainage containers. Vital signs, weight,
intake and output, laboratory values, and patient status are frequently
monitored. The nurse uses a flow sheet to document each exchange and records
vital signs, dialysate concentration, med-ications added, exchange volume,
dwell time, dialysate fluid balance for the exchange (fluid lost or gained),
and cumulative fluid balance. The nurse also carefully assesses skin turgor and
mucous mem-branes to evaluate fluid status and monitor the patient for edema.
If the peritoneal fluid does not drain
properly, the nurse can fa-cilitate drainage by turning the patient from side
to side or raising the head of the bed. The catheter should never be pushed in.
Other measures to promote drainage include checking the patency of the catheter
by inspecting for kinks, closed clamps, or an air lock. The nurse always
monitors for complications, including peritonitis, bleeding, respiratory difficulty,
and leakage of peritoneal fluid. Ab-dominal girth may be measured periodically
to determine if the pa-tient is retaining large amounts of dialysis solution.
Additionally, the nurse must ensure that the peritoneal dialysis catheter
remains secure and that the dressing remains dry. Physical comfort mea-sures,
frequent turning, and skin care are provided. The patient and family are
educated about the procedure and are kept in-formed about progress (fluid loss,
weight loss, laboratory values). Emotional support and encouragement are given
to the patient and family during this stressful and uncertain time.
Continuous Ambulatory Peritoneal Dialysis
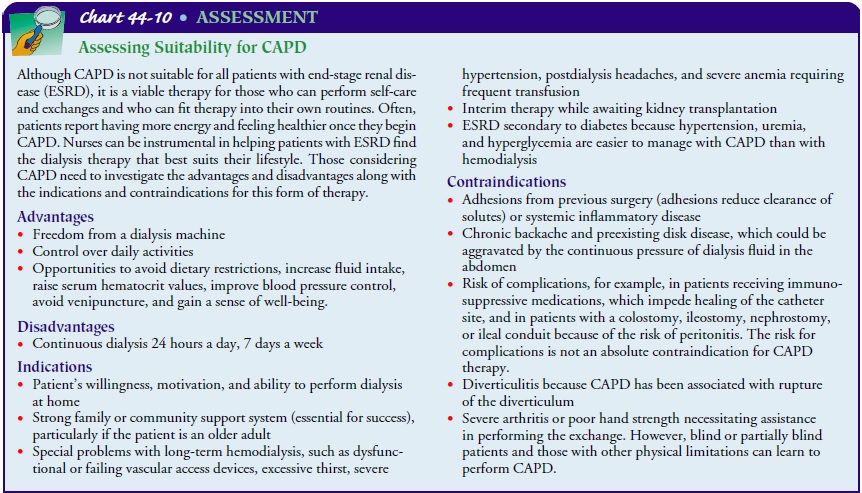
CAPD
is a form of dialysis used for many patients with ESRD. CAPD is performed at
home by the patient or a trained caregiver, who is usually a family member; the
procedure allows the patient reasonable freedom and control of daily activities
(Chart 44-10).
UNDERLYING PRINCIPLES
CAPD
works on the same principles as other forms of peritoneal dialysis: diffusion and
osmosis. Less extreme fluctuations in the patient’s laboratory results occur
with CAPD than with inter-mittent peritoneal dialysis or hemodialysis because
the dialysis is constantly in progress. The serum electrolyte levels usually
remain in the normal range.
PROCEDURE
The
patient performs exchanges four or five times a day, 24 hours a day, 7 days a
week, at intervals scheduled throughout the day (before meals and bedtime).
After infusing the dialysate into the peritoneal cavity through the catheter (over
about 10 minutes), the patient can fold the bag and tuck it underneath the
clothing during the dwell time. This provides the patient with some free-dom
and reduces the number of connections and disconnections necessary at the
catheter end of the tubing, thereby reducing the risk of contamination and
peritonitis.
The longer the dwell time, the better the clearance of middle-sized molecules. It is thought that these molecules may be sig-nificant uremic toxins. At the end of the dwell time, the dialysate is drained from the peritoneal cavity by unfolding the empty bag, opening the clamp, and placing the bag lower than the ab-domen near the floor. This allows the peritoneal fluid to drain out by gravity. When drainage ends, the patient repeats the pro-cedure by spiking a new bag containing dialysate and infusing the solution into the peritoneal cavity. Other systems are avail-able that allow the catheter to be clamped, disconnected, and capped, thus allowing the patient freedom from wearing an empty dialysate bag. Before the next exchange, however, an empty drainage bag must be attached to permit drainage of the dwell solution.
COMPLICATIONS
To
reduce the risk of peritonitis, the patient takes meticulous care to avoid
contaminating the catheter, fluid, or tubing and acci-dentally disconnecting
the catheter from the tubing. The catheter is protected from manipulation, and
the catheter entry site is meticulously cared for according to a standardized
protocol.
Nursing Management
In
addition to the complications of peritoneal dialysis previously described,
patients who elect to use CAPD may experience al-tered body image because of
the abdominal catheter and the bag and tubing. Waist size increases from 1 to 2
inches (or more) with fluid in the abdomen. This affects clothing selection and
may make the patient feel “fat.” Body image may be so altered that pa-tients do
not want to look at or care for the catheter for days or weeks. The nurse may
arrange for the patient to talk with other patients who have adapted well to
CAPD. Although some pa-tients have no psychological problems with the
catheter—they think of it as their lifeline and as a life-sustaining
device—other patients feel they are doing exchanges all day long and have no free
time, particularly in the beginning. They may experience de-pression because
they feel overwhelmed with the responsibility of self-care.
Patients
undergoing CAPD may also experience altered sexu-ality patterns and sexual
dysfunction. The patient and partner may be reluctant to engage in sexual
activities, partly because of the catheter being psychologically “in the way”
of sexual perfor-mance. The peritoneal catheter, drainage bag, and about 2 L of
dialysate may interfere with the patient’s sexual function and body image as
well. Although these problems may resolve with time, some problems may warrant
special counseling. Questions by the nurse about concerns related to sexuality
and sexual function often provide the patient with a welcome opportunity to
dis-cuss these issues and a first step toward their resolution.
PROMOTING HOME AND COMMUNITY-BASED CARE
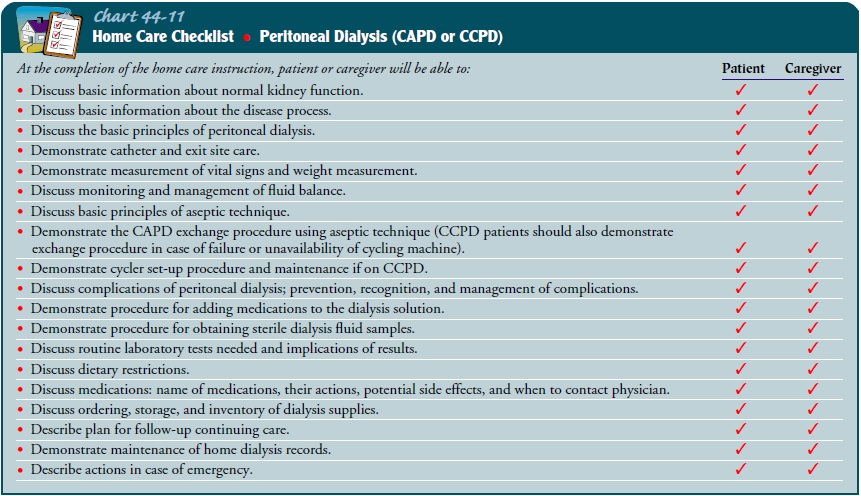
Teaching Patients Self-Care.
Patients are
taught as inpatients oroutpatients to perform CAPD once their condition is
medically stable. Training usually takes 5 days to 2 weeks. Patients are taught
according to their own learning ability and knowledge level, and only as much
at one time as they can handle without feeling uncomfortable or becoming
overwhelmed. Education topics for the patient and family who will be performing
peri-toneal dialysis at home are described in Chart 44-11.
Because
of protein loss with continuous peritoneal dialysis, pa-tients are instructed
to eat a high-protein, well-balanced diet. They are also encouraged to increase
their daily fiber intake to help prevent constipation, which can impede the
flow of dialysate into or out of the peritoneal cavity. Many patients gain 3 to
5 lb within a month of initiating CAPD, so they may be asked to limit their
carbohydrate intake to avoid excessive weight gain. Potas-sium, sodium, and
fluid restrictions are not usually needed. Patients commonly lose about 2 L of
fluid over and above the 8 L of dialysate infused into the abdomen during a
24-hour period, permitting a normal fluid intake even in an anephric patient (a
patient without kidneys). Greater small-solute clearances are associated with
better dietary intake and better nutrition (Wang, Sea, Ip et al., 2001).
Continuing Care.
Follow-up care through phone calls, visits tothe outpatient department, and continuing home care assists pa-tients in the transition to home and promotes their active partic-ipation in their own health care. Patients often depend on checking with the nurse to see if they are making the right choices about dialysate or control of blood pressure, or simply to discuss a problem.
Patients
may be seen by the CAPD team as outpatients once a month or more often if
needed. The exchange procedure is eval-uated at that time to see that strict
aseptic technique is being used. The CAPD nurse may change the tubing used to
instill the dialysate every 4 to 8 weeks. Long-life tubing now lasts up to 6
months before tubing changes are necessary. Infrequent tubing changes decrease
the risk of contamination. Blood chemistry val-ues are followed closely to make
certain the therapy is adequate for the patient.
If a
referral is made for home care, the home care nurse assesses the home
environment and suggests modifications to accommo-date the equipment and
facilities needed to carry out CAPD. In addition, the nurse assesses the
patient’s and family’s under-standing of CAPD and their use of safe technique
in performing CAPD. Additional assessments include checking for changes related
to renal disease, complications such as peritonitis, and treatment-related
problems such as heart failure, inadequate drainage, and weight gain or loss.
The nurse continues to reinforce and clarify teaching about CAPD and renal
disease and assesses the patient’s and family’s progress in coping with the
procedure. In addition, the patient is reminded about the need to participate
in health promotion activities and health screening.
Due to
the projected high numbers of elderly patients who will develop ESRD in the
future, the nursing home or extended-care facility will become an increasingly
important site for both rehabilitation and long-term management of patients
with renal failure. Although few such sites currently provide dialysis, highly
structured educational programs for personnel in these environ-ments by
nephrology staff will likely make these effective sites of care for patients
requiring continuous peritoneal dialysis (Carey, Chorney, Pherson et al.,
2001).
Continuous Cyclic Peritoneal Dialysis
CCPD
combines overnight intermittent peritoneal dialysis with a prolonged dwell time
during the day. The peritoneal catheter is connected to a cycler machine every
evening, and the patient re-ceives three to five 2-L exchanges during the
night. In the morn-ing, the patient caps off the catheter after infusing 1 to 2
L of fresh dialysate. This dialysate remains in the abdominal cavity until the
tubing is reattached to the cycler machine at bedtime. Because the machine is
very quiet, the patient can sleep. Moreover, the extra-long tubing allows the
patient to move and turn normally during sleep.
CCPD
has a lower infection rate than other forms of peri-toneal dialysis because
there are fewer opportunities for contam-ination with bag changes and tubing
disconnections. It also allows the patient to be free from exchanges throughout
the day, making it possible to work more freely and carry out activities of
daily living.
Related Topics