Chapter: Medical Surgical Nursing: Management of Patients With Upper or Lower Urinary Tract Dysfunction
Hemodialysis - Dialysis
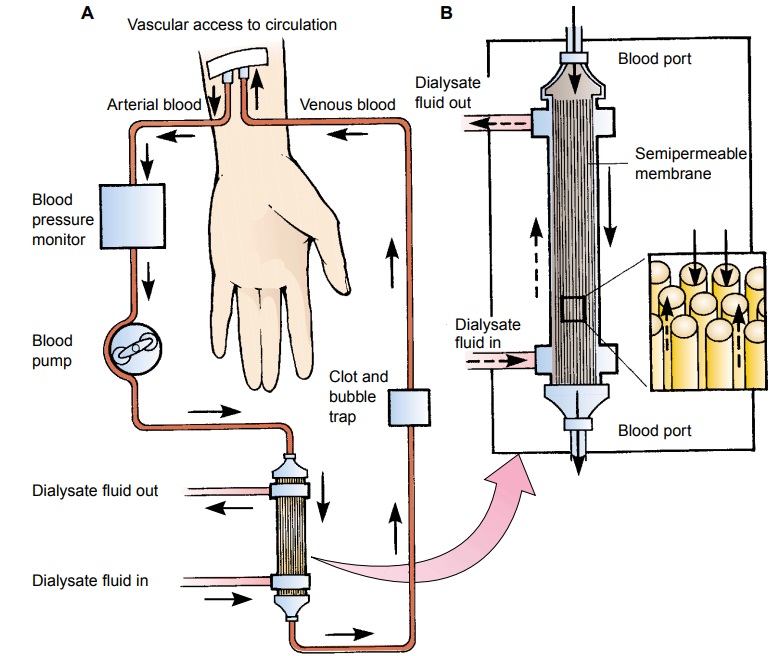
HEMODIALYSIS
Hemodialysis
is the most commonly used method of dialysis: more than 300,000 Americans
currently receive hemodialysis (Parker, Bliwise & Rye, 2000). It is used
for patients who are acutely ill and require short-term dialysis (days to
weeks) and for patients with ESRD who require long-term or permanent ther-apy.
A dialyzer (once referred to as an
artificial kidney) serves as a synthetic semipermeable membrane, replacing the
renal glo-meruli and tubules as the filter for the impaired kidneys.
For
patients with chronic renal failure, hemodialysis prevents death, although it
does not cure renal disease and does not com-pensate for the loss of endocrine
or metabolic activities of the kid-neys. Patients receiving hemodialysis must
undergo treatment for the rest of their lives or until they undergo a successful
kidney transplant. Treatments usually occur three times a week for at least 3
to 4 hours per treatment (some patients undergo short-daily hemodialysis; Chart
44-7). Patients receive chronic or mainte-nance dialysis when they require
dialysis therapy for survival and control of uremic symptoms. The trend in
managing ESRD is to initiate treatment before the signs and symptoms associated
with uremia become severe.
Principles of Hemodialysis
The
objectives of hemodialysis are to extract toxic nitrogenous substances from the
blood and to remove excess water. In he-modialysis, the blood, laden with
toxins and nitrogenous wastes, is diverted from the patient to a machine, a
dialyzer, in which the blood is cleansed and then returned to the patient.
Diffusion,
osmosis, and ultrafiltration are
the principles onwhich hemodialysis is based. The toxins and wastes in the
blood are removed by diffusion—that is, they move from an area of higher
concentration in the blood to an area of lower concentra-tion in the dialysate. The dialysate is a solution
made up of all the important electrolytes in their ideal extracellular
concentra-tions. The electrolyte level in the patient’s blood can be brought
under control by properly adjusting the dialysate bath. The semi-permeable
membrane impedes the diffusion of large molecules, such as red blood cells and
proteins.
Excess water is removed from the blood by osmosis, in which water moves from an area of higher solute concentration (the blood) to an area of lower solute concentration (the dialysate bath). Ultrafiltration is defined as water moving under high pressure to an area of lower pressure. This process is much more efficient at water removal than osmosis. Ultrafiltration is ac-complished by applying negative pressure or a suctioning force to the dialysis membrane. Because patients with renal disease usu-ally cannot excrete water, this force is necessary to remove fluid to achieve fluid balance.
The
body’s buffer system is maintained using a dialysate bath made up of
bicarbonate (most common) or acetate, which is me-tabolized to form
bicarbonate. The anticoagulant heparin is ad-ministered to keep blood from
clotting in the dialysis circuit. Cleansed blood is returned to the body. By
the end of the dialy-sis treatment, many waste products have been removed, the
electrolyte balance has been restored to normal, and the buffer sys-tem has
been replenished.
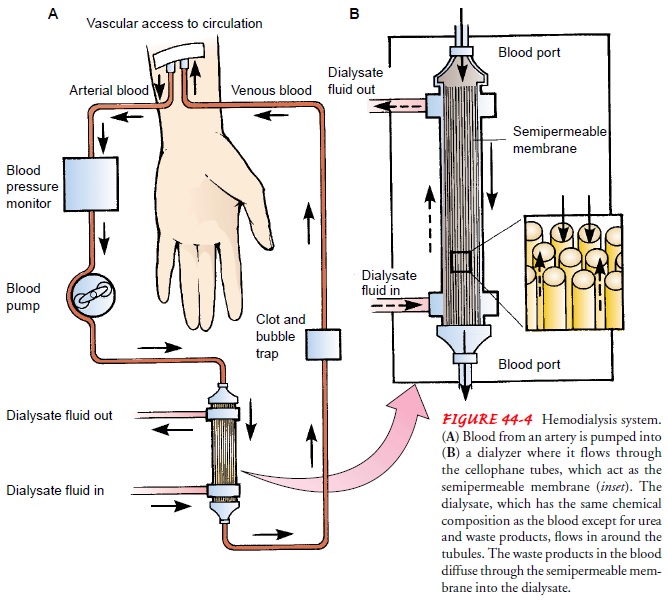
Equipment: Dialyzers
Most
dialyzers, or artificial kidneys, are either flat-plate dialyzers or
hollow-fiber artificial kidneys that contain thousands of tiny cellophane
tubules that act as semipermeable membranes. The blood flows through the
tubules, while a solution (the dialysate) circulates around the tubules. The
exchange of wastes from the blood to the dialysate occurs through the
semipermeable mem-brane of the tubules (Fig. 44-4).
Dialyzers
have undergone many technological changes. The difference between flat-plate
dialyzers and hollow-fiber dialyzers lies in performance and biocompatibility.
Biocompatibility refers to the ability of the dialyzer to accomplish its
objectives without causing hypersensitive, allergic, or adverse reactions. Some
dia-lyzers remove middle-weight molecules at a faster rate and ultra-filtrate
at higher rates, which is thought to reduce neuropathy of the lower
extremities, a complication of long-term hemodialysis. In general, the more
efficient the dialyzer, the higher the cost.
Another technological advance is high-flux dialysis, which uses highly permeable membranes that increase the clearance of low- and mid-molecular-weight molecules. These special mem-branes are used with higher-than-traditional rates of flow for the blood entering and exiting the dialyzer (500 to 800 mL/min). High-flux dialysis requires the use of precise volumetric ultra-filtration control systems, and not every dialysis unit can perform this type of dialysis. High-flux dialysis increases the efficiency of treatments while shortening their duration and reducing the need for heparin.
Because
of the costs associated with hemodialysis, hemodi-alyzers are commonly reused
in dialysis centers in the United States. Recent studies have raised concerns
about the mortality risks associated with some hemodialyzer reuse practices.
Results from the United States Renal Data System (USRDS) Dialysis Morbidity and
Mortality Study demonstrated differences in mor-tality rate with the reuse of
certain hemodialyzers. Among all membranes, mortality is lowest for patients
treated with high-flux synthetic membranes. The bleaching process to reuse
high-flux synthetic membrane dialyzers may account for the lower mortal-ity
rate: with this process, clearance of larger molecules is still pos-sible, even
though the hemodialyzer is not new. These findings are important because
high-flux hemodialyzers are very efficient and are used in most dialysis
centers (Port, Wolfe, Hulbert-Shear-son et al., 2001).
Vascular Access
Access
to the patient’s vascular system must be established to allow blood to be
removed, cleansed, and returned to the pa-tient’s vascular system at rates
between 200 and 800 mL/minute. Several types of access are available.
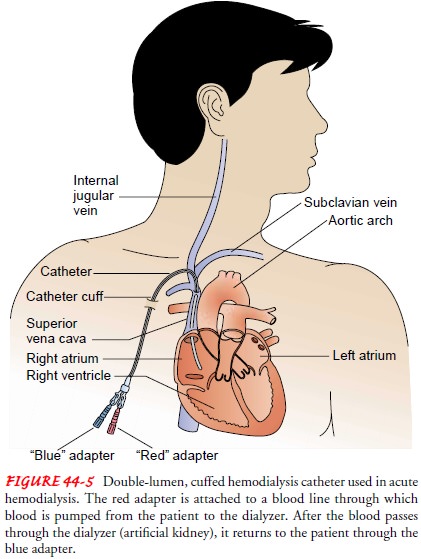
SUBCLAVIAN, INTERNAL, JUGULAR, AND FEMORAL CATHETERS
Immediate
access to the patient’s circulation for acute hemodial-ysis is achieved by
inserting a double-lumen or multilumen cath-eter into the subclavian, internal
jugular, or femoral vein. Although this method of vascular access involves some
risk (eg, hematoma, pneumothorax, infection, thrombosis of the subclavian vein,
and inadequate flow), it can be used for several weeks. The catheters are
removed when no longer needed, because the patient’s condi-tion has improved or
another type of access has been established. Double-lumen, cuffed catheters may
also be surgically inserted into the subclavian vein of patients requiring a
central venous catheter for dialysis (Fig. 44-5).
FISTULA
A more
permanent access, known as a fistula,
is created surgically (usually in the forearm) by joining (anastomosing) an
artery to a vein, either side to side or end to side (Fig. 44-6). Needles are
in-serted into the vessel to obtain blood flow adequate to pass through the
dialyzer. The arterial segment of the fistula is used for arterial flow and the
venous segment for reinfusion of the di-alyzed blood. The fistula takes 4 to 6
weeks to mature before it is ready for use. This gives time for healing and for
the venous seg-ment of the fistula to dilate to accommodate two large-bore
(14-or 16-gauge) needles. The patient is encouraged to perform exer-cises to
increase the size of these vessels (ie, squeezing a rubber ball for forearm
fistulas) and thereby to accommodate the large-bore needles used in
hemodialysis.
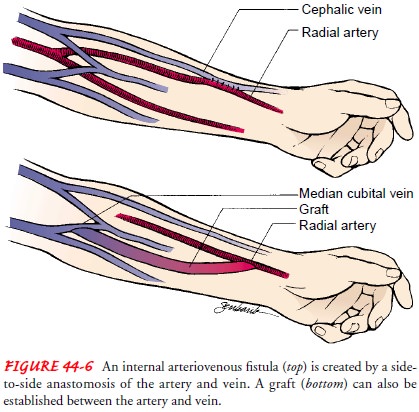
GRAFT
An arteriovenous graft can be created by subcutaneously inter-posing a biologic, semibiologic, or synthetic graft material be-tween an artery and vein (see Fig. 44-6). The most commonly used synthetic graft material is expanded polytetrafluoroethylene (PTFE). Usually, a graft is created when the patient’s vessels are not suitable for a fistula. Patients with compromised vascular sys-tems (eg, from diabetes) often need to have a graft to undergo hemodialysis. Grafts are usually placed in the forearm, upper arm, or upper thigh. Infection and thrombosis are the most common complications of arteriovenous grafts.
Complications of Hemodialysis
Although
hemodialysis can prolong life indefinitely, it does not alter the natural
course of the underlying kidney disease, nor does it completely replace kidney
function. The patient is subject to a number of problems and complications. One
leading cause of death among patients undergoing maintenance hemodialysis is
atherosclerotic cardiovascular disease. Disturbances of lipid me-tabolism
(hypertriglyceridemia) appear to be accentuated by hemo-dialysis. Heart
failure, coronary heart disease and anginal pain, stroke, and peripheral
vascular insufficiency may occur and may incapacitate the patient. Anemia and
fatigue contribute to di-minished physical and emotional well-being, lack of
energy and drive, and loss of interest, although the use of erythropoietin
(Epogen) before the start of dialysis has been shown to have a sig-nificant
effect on hematocrit values for the first 19 months after starting dialysis (Fink
et al., 2001). Increased dialyzer clotting may occur, which is prevented by
adjusting heparin doses, and dialyzer solute clearances may decrease slightly
(Eschbach & Adamson, 1989).
Gastric ulcers and other gastrointestinal problems occur from the physiologic stress of chronic illness, medication, and related problems. Disturbed calcium metabolism leads to renal osteo-dystrophy that produces bone pain and fractures. Other problems include fluid overload associated with heart failure, malnutrition, infection, neuropathy, and pruritus.
Up to
85% of people undergoing hemodialysis experience major sleep problems that
further complicate their overall health status. Recent studies suggest that
early-morning or late-afternoon dialysis may be a risk factor for developing
sleep abnormalities. Researchers suggest such interventions as changing the
tempera-ture of the dialysate bath to prevent temperature elevation and
limiting napping during dialysis as strategies to reduce sleep prob-lems in
individuals receiving hemodialysis (Parker et al., 2000). Other complications
of dialysis treatment may include the following:
· Hypotension may occur
during the treatment as fluid is re-moved. Nausea and vomiting, diaphoresis,
tachycardia, and dizziness are common signs of hypotension.
· Painful muscle cramping
may occur, usually late in dialysis as fluid and electrolytes rapidly leave the
extracellular space.
· Exsanguination may occur
if blood lines separate or dialy-sis needles accidentally become dislodged.
· Dysrhythmias may result
from electrolyte and pH changes or from removal of antiarrhythmic medications
during dialysis.
· Air embolism is rare but
can occur if air enters the vascular system.
· Chest pain may occur in
patients with anemia or arterioscle-rotic heart disease.
· Dialysis disequilibrium
results from cerebral fluid shifts. Signs and symptoms include headache, nausea
and vom-iting, restlessness, decreased level of consciousness, and seizures. It
is more likely to occur in acute renal failure or when blood urea nitrogen
levels are very high (exceeding 150 mg/dL).
Long-Term Management
During
dialysis, the patient, the dialyzer, and the dialysate bath require constant
monitoring because numerous complications are possible, including air embolism,
inadequate or excessive ultrafiltration (hypotension, cramping, vomiting),
blood leaks, con-tamination, and access complications. The nurse in the
dialysis unit has an important role in monitoring, supporting, assessing, and
educating the patient. Nursing care of the patient and main-tenance of the
access device are discussed under “Care of the Hos-pitalized Dialysis Patient.”
Pharmacologic Therapy
Just
as many medications are excreted wholly or in part by the kidneys, many
medications are removed from the blood during hemodialysis; therefore, the
physician may need to adjust the dosage. Metabolites of drugs that are bound to
protein are not re-moved during dialysis. Removal of other drug metabolites
de-pends on the weight and size of the molecule.
Patients
undergoing hemodialysis who require medications (eg, cardiac glycosides,
antibiotic agents, antiarrhythmic medica-tions, antihypertensive agents) are
monitored closely to ensure that blood and tissue levels of these medications
are maintained without toxic accumulation.
In
patients receiving dialysis, all medications and their dosages must be
carefully evaluated. Antihypertensive therapy, often part of the dialysis
patient’s regimen, is one example in which com-munication, teaching, and
evaluation can make a difference in patient outcomes. The patient must know
when and when not to take the medication. For example, if an antihypertensive
agent is taken on a dialysis day, a hypotensive effect may occur during
dialysis, causing dangerously low blood pressure. Many medica-tions that are
taken once daily can be held until after the dialysis treatment.
Nutritional and Fluid Therapy
When
damaged kidneys cannot excrete end products of metabo-lism, these substances
accumulate in the serum as toxins. The re-sulting symptoms, collectively known
as uremic symptoms or uremic syndrome, affect every body system. The more
toxins that accumulate, the more severe the symptoms.
Diet
is an important factor for patients on hemodialysis be-cause of the effects of
uremia. Goals of nutritional therapy are to minimize uremic symptoms and fluid
and electrolyte imbalances; to maintain good nutritional status through
adequate protein, calorie, vitamin, and mineral intake; and to enable the
patient to eat a palatable and enjoyable diet. Restricting dietary protein
de-creases the accumulation of nitrogenous wastes, reduces uremic symptoms, and
may even postpone the initiation of dialysis for a few months. Restriction of
fluid is also part of the dietary pre-scription because fluid accumulation may
occur, leading to weight gain, heart failure, and pulmonary edema.
With
the initiation of hemodialysis, the patient’s dietary intake usually still
requires some restriction of dietary protein, sodium, potassium, and fluid
intake. Protein intake is restricted to about 1 g/kg ideal body weight per day;
therefore, protein must be of high biologic quality and consist of the
essential amino acids to prevent poor protein use and to maintain a positive
nitrogen bal-ance. Examples of foods high in biologic protein content include
eggs, meat, milk, poultry, and fish. Sodium is usually restricted to 2 to 3
g/day; fluids are restricted to an amount equal to the daily urine output plus
500 mL/day. The goal for hemodialysis patients is to keep their interdialytic
(between dialysis treatments) weight gain under 1.5 kg. Potassium restriction
(average 1.5 to 2.5 g/day) depends on the amount of residual renal function and
the frequency of dialysis (National Kidney Foundation, 2000).
Dietary
restriction is an unwelcome change in lifestyle for many patients with chronic
renal failure. Patients often feel stig-matized in social situations because
there may be few food selec-tions available for their diet. If the restrictions
are ignored, life-threatening complications, such as hyperkalemia and
pul-monary edema, may result. Thus, the patient may feel punished for
responding to basic human drives to eat and drink. The nurse who encounters a
patient with symptoms or complications re-sulting from dietary indiscretion must
avoid harsh, judgmental, or punitive tones when communicating with him or her.
Nursing Management
Patients
requiring long-term hemodialysis are often concerned about the unpredictability
of the illness and the disruption of their lives. They often have financial
problems, difficulty holding a job, waning sexual desire and impotence,
depression from being chronically ill, and fear of dying. Younger patients
worry about marriage, having children, and the burden that they bring to their
families. The regimented lifestyle that frequent dialysis treat-ments and
restrictions in food and fluid intake impose is often de-moralizing to the
patient and family.
MEETING PSYCHOSOCIAL NEEDS
Dialysis
alters the lifestyle of the patient and family. The amount of time required for
dialysis and physician visits and being chron-ically ill can create conflict,
frustration, guilt, and depression. It may be difficult for the patient,
spouse, and family to express anger and negative feelings.
The
nurse needs to give the patient and family the opportu-nity to express feelings
of anger and concern over the limitations that the disease and treatment impose
and over possible financial problems and job insecurity. If anger is not
expressed, it may be directed inward and lead to depression, despair, and
attempts at suicide (suicide is more prevalent in dialysis patients); however,
if anger is projected outward to other people, it may destroy al-ready
threatened family relationships.
Although
normal in this situation, these feelings are often pro-found and overwhelming.
Counseling and psychotherapy may be necessary. Depression may require treatment
with antidepressant agents. Referring the patient and family to a mental health
pro-vider with expertise in the care of patients receiving dialysis may also be
helpful. Clinical nurse specialists, psychologists, and social workers may be
helpful in assisting the patient and family to cope with the changes brought
about by renal failure and its treatment.
The
sense of loss that the patient experiences cannot be under-estimated because
every aspect of a “normal life” is disrupted. Some patients use denial to deal
with the overwhelming array of medical problems (eg, infections, hypertension,
anemia, neuro-pathy). Staff who are tempted to label the patient as
noncompli-ant must consider the impact of renal failure and its treatment on
the patient and family and the coping strategies that they may use. The nurse
helps the patient to identify safe, effective coping strategies to cope with
these ever-present problems and fears (Tonelli et al., 2001).
PROMOTING HOME AND COMMUNITY-BASED CARE
Preparing
a patient for hemodialysis is challenging. Often the patient does not fully
comprehend the impact of dialysis, and learning needs may go unrecognized. Good
communication be-tween the dialysis staff (in the hospital and outpatient
clinic), unit staff, and home care nurses is essential for providing sound,
continuous care.
Teaching Patients Self-Care.
Assessment
helps identify thelearning needs of the patient and family members. In many
cases, the patient is home before learning needs and readiness to learn can be
thoroughly evaluated; therefore, hospital-based nurses, dialysis staff, and
home care nurses must work together to pro-vide appropriate teaching that meets
the patient’s and family’s changing needs and readiness to learn.
The
diagnosis of chronic renal failure and the need for dialy-sis often overwhelm
the patient and family. In addition, many patients with ESRD have depressed
mentation, a shortened at-tention span, a decreased level of concentration, and
altered per-ceptual states. Therefore, teaching must occur in brief, 10- to
15-minute sessions, with time added for clarification, repetition,
reinforcement, and questions from the patient and family. The nurse needs to
convey a nonjudgmental attitude to enable the pa-tient and family to discuss
options and their feelings about those options. Team conferences are helpful
for sharing information and providing every team member the opportunity to discuss
the needs of the patient and family.
Teaching Patients About Hemodialysis.
Although most patientswho require hemodialysis undergo the procedure in
an outpatient setting, home hemodialysis is an option for some. Home
hemo-dialysis requires a highly motivated patient who is willing to take
responsibility for the procedure and is able to adjust each treat-ment to meet
the body’s changing needs. It also requires the commitment and cooperation of a
family member to assist the patient. Many patients, however, are not
comfortable imposing on others in that way and do not wish to subject family
members to the feeling that their home is being turned into a clinic.
The
health care team should never force a patient into using home hemodialysis.
Because this treatment requires many signif-icant changes in the home and
family, home hemodialysis must be the patient’s and family’s decision.
The
patient undergoing home hemodialysis and the caregiver assisting that patient
must be trained to prepare, operate, and dis-assemble the dialysis machine;
maintain and clean the equip-ment; administer medications (eg, heparin) into
the machine lines; and handle emergency problems (hemodialysis dialyzer
rupture, electrical or mechanical problems, hypotension, shock, and seizures). Because
home hemodialysis places primary re-sponsibility for the treatment on the
patient and the family mem-ber, they must understand and be capable of
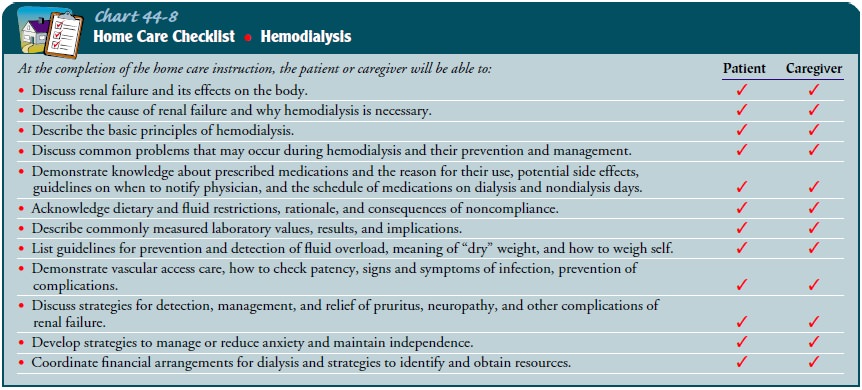
performing all aspects of the hemodialysis procedure (Chart 44-8).
Before
home hemodialysis is initiated, the home environment, household and community
resources, and ability and willingness of the patient and family to carry out
this treatment are assessed. The home is surveyed to see if electrical outlets,
plumbing facilities, and storage space are adequate. Modifications may be
needed to enable the patient and assistant to perform dialysis safely and to
deal with emergencies.
Once
home dialysis is initiated, the home care nurse must visit periodically to
evaluate compliance with the recommended tech-niques, to assess the patient for
complications, to reinforce previ-ous teaching, and to provide reassurance.
Continuing Care.
The health care team’s goal in treating patientswith chronic renal failure is to maximize their vocational poten-tial, functional status, and quality of life. To facilitate renal reha-bilitation, appropriate follow-up and monitoring by members of the health care team (physicians, dialysis nurses, social workers, psychologist, home care nurses, and others as appropriate) are essential to identify and resolve problems early on. Many patients with chronic renal failure can resume relatively normal lives, doing the things that are important to them: traveling, exercising, working, or actively participating in family activities.
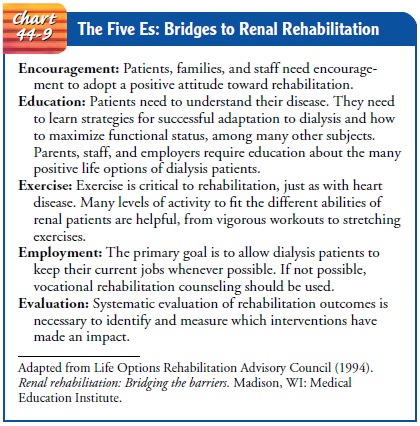
If appro-priate interventions are available early in the course of dialysis, the potential for better health improves, and the patient can remain active in family and community life. Chart 44-9 outlines the es-sential elements identified by the Life Options Rehabilitation Advisory Council for the rehabilitation of dialysis patients. Out-come goals for renal rehabilitation include employment for those able to work, improved physical functioning of all patients, im-proved understanding about adaptation and options for living well, increased control over the effects of kidney disease and dial-ysis, and resumption of activities enjoyed before dialysis.
Related Topics