Chapter: Medical Surgical Nursing: Management of Patients With Upper or Lower Urinary Tract Dysfunction
Neurogenic Bladder - Dysfunctional Voiding Patterns
NEUROGENIC
BLADDER
Neurogenic bladder is a dysfunction that results from a lesion
ofthe nervous system. It may be caused by spinal cord injury, spinal tumor,
herniated vertebral disk, multiple sclerosis, congenital anomalies (spina
bifida or myelomeningocele), infection, or dia-betes mellitus.
Pathophysiology
The
two types of neurogenic bladder are spastic (or reflex) blad-der and flaccid
bladder. Spastic bladder is the more common type and is caused by any spinal
cord lesion above the voiding reflex arc (upper motor neuron lesion). The
result is a loss of conscious sensation and cerebral motor control. A spastic
bladder empties on reflex, with minimal or no controlling influence to regulate
its activity.
Flaccid
bladder is caused by a lower motor neuron lesion, commonly resulting from
trauma. This form of neurogenic blad-der has increasingly been recognized as a
problem in patients with diabetes mellitus. The bladder continues to fill and
becomes greatly distended, and overflow incontinence occurs. The blad-der
muscle does not contract forcefully at any time. Because sen-sory loss may accompany
a flaccid bladder, the patient feels no discomfort.
Assessment and Diagnostic Findings
Evaluation
for neurogenic bladder involves measurement of fluid intake, urine output, and
residual urine volume; urinalysis; and assessment of sensory awareness of
bladder fullness and de-gree of motor control. Comprehensive urodynamic studies
are also performed.
Complications
The
most common complication of neurogenic bladder is infec-tion resulting from
urinary stasis and catheterization. Urolithia-sis (stones in the urinary tract)
may develop from urinary stasis, infection, or demineralization of bone from
prolonged immobilization. Renal failure can also occur from vesicoureteral
reflux (backward flow of retained urine from the bladder into the ureters) with
eventual hydronephrosis (dilation of the pelvis of the kidney resulting from
obstruction to the flow of urine) and atrophy of the kidney. Indeed, renal
failure is the major cause of death of pa-tients with neurologic impairment of
the bladder.
Medical Management
The
problems resulting from neurogenic bladder disorders vary considerably from
patient to patient and are a major challenge to the health care team. There are
several long-term objectives ap-propriate for all types of neurogenic bladders:
· Preventing
overdistention of the bladder
· Emptying the bladder
regularly and completely
· Maintaining urine
sterility with no stone formation
· Maintaining adequate
bladder capacity with no reflux
Specific
interventions include continuous, intermittent, or self-catheterization, use of
an ex-ternal condom-type catheter, a diet low in calcium (to prevent calculi),
and encouragement of mobility and ambulation. A lib-eral fluid intake is
encouraged to reduce the urinary bacterial count, reduce stasis, decrease the concentration
of calcium in the urine, and minimize the precipitation of urinary crystals and
sub-sequent stone formation.
To
further enhance bladder emptying of a flaccid bladder, the individual may try
“double voiding.” After each voiding, the in-dividual remains on the toilet,
relaxes for 1 to 2 minutes, and then attempts to void again in an effort to
further empty the bladder. This can be effective in patients with disorders
characterized by neurogenic bladder (eg, multiple sclerosis) (Halper, 1998).
Use of
timed, or habit, voiding is also considered. For exam-ple, a 2-hour voiding
schedule may be established to prevent overdistention. A bladder retraining
program may be effective in treating a spastic bladder or urine retention
(Davies et al., 2000; Joseph, 1999).
PHARMACOLOGIC THERAPY
Parasympathomimetic
medications, such as bethanechol (Ure-choline), may help to increase the
contraction of the detrusor muscle.
SURGICAL MANAGEMENT
In
some cases, surgery may be carried out to correct bladder neck contractures or
vesicoureteral reflux or to perform some type of urinary diversion procedure.
CATHETERIZATION
In
patients with a urologic disorder or with marginal kidney func-tion, care must
be taken to ensure that urinary drainage is ade-quate and that kidney function
is preserved. When urine cannot be eliminated naturally and must be drained
artificially, catheters may be inserted directly into the bladder, the ureter,
or the renal pelvis. Catheters vary in size, shape, length, material, and
config-uration. The type of catheter used depends on its
purpose.Catheterization is performed to achieve the following:
· Relieve urinary tract
obstruction
·
Assist with postoperative drainage in urologic and
other surgeries
· Provide a means to
monitor accurate urine output in criti-cally ill patients
· Promote urinary drainage
in patients with neurogenic blad-der dysfunction or urine retention
· Prevent urinary leakage
in patients with stage III to IV pres-sure ulcers
A
patient should be catheterized only if necessary because catheterization
commonly leads to urinary tract infection. In ad-dition, urinary catheters have
been associated with other compli-cations, such as bladder spasms, urethral
strictures, and pressure necrosis.
Indwelling Devices and Infections.
When an indwelling
cath-eter cannot be avoided, a closed drainage system is essential. This
drainage system is designed to prevent any disconnections, thereby reducing the
risk of contamination. One common system consists of an indwelling catheter, a
connecting tube, and a collecting bag with an antireflux chamber emptied by a
drainage spout. Another common system has a triple-lumen indwelling urethral
catheter attached to a closed sterile drainage system. With the triple-lumen
catheter, urinary drainage occurs through one channel. The re-tention balloon
of the catheter is inflated with water or air through the second channel, and
the bladder is continuously irrigated with sterile irrigating solution through
the third channel. Triple-lumen catheters are commonly used after transurethral
prostate surgery.
An
indwelling catheter can lead to infection. Bacterial colo-nization
(bacteriuria) occurs within 2 weeks in half of catheter-ized patients and
within 4 to 6 weeks in almost all patients after insertion of a catheter—even
if recommendations for infection control and catheter care are followed
carefully.
Urinary
tract infections are the most commonly occurring nosocomial infections,
accounting for 40% of them. Every year, about 1 million patients in acute-care
hospitals develop nosoco-mial urinary tract infections, and about 80% of these
are associ-ated with the use of indwelling urinary catheters (Phillips, 2000).
Most urinary tract infections follow instrumentation of the uri-nary tract,
usually catheterization. The pathogens responsible for catheter-associated
urinary tract infections include Escherichiacoli
and Klebsiella, Proteus, Pseudomonas,
Enterobacter, Serratia, and Candida
species. Many of these organisms are part of the pa-tient’s endogenous or
normal bowel flora or are acquired through cross-contamination by patients or
health care personnel or through exposure to nonsterile equipment.
Catheters
impede most of the natural defenses of the lower urinary tract by obstructing
the periurethral ducts, irritating the bladder mucosa, and providing an
artificial route for organisms to enter the bladder. Organisms may be
introduced from the ure-thra into the bladder during catheterization, or they
may migrate along the epithelial surface of the urethra or external surface of
the catheter.
The
spout of the urinary drainage bag can become contami-nated when opened to drain
the bag. Bacteria enter the urinary drainage bag, multiply rapidly, and then
migrate to the drainage tubing, catheter, and bladder. Scanning electron
microscopy has demonstrated that thick layers (biofilms) of organisms often
colonize the internal surfaces of catheters and drainage systems (Doyle et al.,
2001; Godfrey & Evans, 2000; Phillips, 2000).
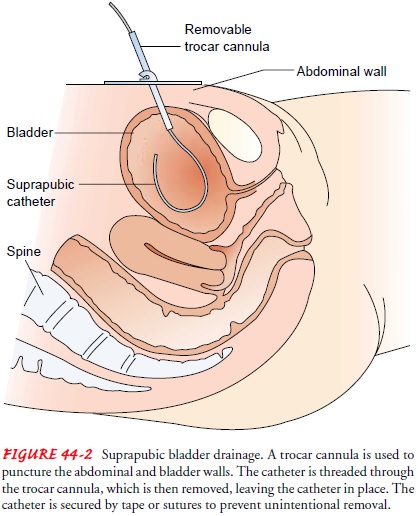
Suprapubic Catheterization.
Suprapubic catheterization allowsbladder drainage by inserting a catheter or tube into the bladder through a suprapubic (above the pubis) incision or puncture (Fig. 44-2).
It may be a temporary measure to divert the flow of urine
from the urethra when the urethral route is impassable (be-cause of injuries,
strictures, prostatic obstruction), after gyneco-logic or other abdominal
surgery when bladder dysfunction is likely to occur, and occasionally after
pelvic fractures. Suprapu-bic catheters may also be used on a long-term basis
for women with urethral destruction secondary to long-term indwelling ure-thral
catheters (Addison, 1999a, 1999b).
For
insertion of the suprapubic catheter, the patient is placed in a supine
position and the bladder distended by administering oral or intravenous fluids
or by instilling sterile saline solution into the bladder through a urethral
catheter. These measures make it easier to locate the bladder. The suprapubic
area is prepared as for surgery and the puncture site located about 5 cm (2 in)
above the symphysis pubis. The bladder is entered through an incision or
through a puncture made by a small trocar (pointed instrument). The catheter or
suprapubic drainage tube is threaded into the blad-der and secured with sutures
or tape; the area around the catheter is covered with a sterile dressing. The
catheter is connected to a sterile closed drainage system, and the tubing is
secured to pre-vent tension on the catheter.
Suprapubic
bladder drainage may be maintained continuously for several weeks. When the
patient’s ability to void is to be tested, the catheter is clamped for 4 hours,
during which time the patient attempts to void. After the patient voids, the
catheter is un-clamped, and the residual urine (the amount of urine remaining)
is measured. If the amount of residual urine is less than 100 mL on two
separate occasions (morning and evening), the catheter is usually removed. If
the patient complains of pain or discomfort, however, the suprapubic catheter
is usually left in place until the patient can void successfully. When a
suprapubic catheter remains in place indefinitely, it is changed regularly at
6- to 12-week in-tervals (Gujral et al., 1999).
Suprapubic
drainage offers certain advantages. Patients can usually void sooner after
surgery than those with urethral cath-eters, and they may be more comfortable.
The catheter allows greater mobility, permits measurement of residual urine
without urethral instrumentation, and presents less risk of bladder infec-tion.
The suprapubic catheter is removed when it is no longer necessary, and a
sterile dressing is placed over the site.
The
patient requires liberal amounts of fluid to prevent en-crustation around the
catheter. Other potential problems include the formation of bladder stones,
acute and chronic infections, and problems collecting urine. An enterostomal
therapist may be con-sulted to assist the patient and family in selecting the
most suitable urine collection system and to teach them about its use and care.
Nursing Management During Catheterization
ASSESSING THE PATIENT AND THE SYSTEM
For
patients with indwelling catheters, the nurse assesses the drainage system to
ensure that it provides adequate urinary drain-age. The color, odor, and volume
of urine are also monitored. An accurate record of fluid intake and urine
output provides essen-tial information about the adequacy of renal function and
urinary drainage.
The
nurse observes the catheter to make sure that it is prop-erly anchored, to
prevent pressure on the urethra at the peno-scrotal junction in male patients,
and to prevent tension and traction on the bladder in both male and female
patients.
Patients
at high risk for urinary tract infection from catheter-ization need to be
identified and monitored carefully. These in-clude women, older adults, and
patients who are debilitated, malnourished, chronically ill, immunosuppressed,
or diabetic. They are observed for signs and symptoms of urinary tract
infec-tion: cloudy malodorous urine, hematuria, fever, chills, anorexia, and
malaise. The area around the urethral orifice is observed for drainage and
excoriation. Urine cultures provide the most accu-rate means of assessing a
patient for infection.
Bladder
ultrasonography can be used for noninvasive mea-surement of bladder volume. A
portable bladder scan can be per-formed to assess the volume of urine in the
bladder, the degree of bladder emptying, and therefore the need for
catheterization (Phillips, 2000; Schott-Baer & Reaume, 2001).
ASSESSING FOR AGE-RELATED COMPLICATIONS
Elderly
patients with an indwelling catheter may not exhibit the typical signs and
symptoms of infection. Therefore, any subtle change in physical condition or
mental status must be considered a possible indication of infection and
promptly investigated because sepsis may occur before the infection is
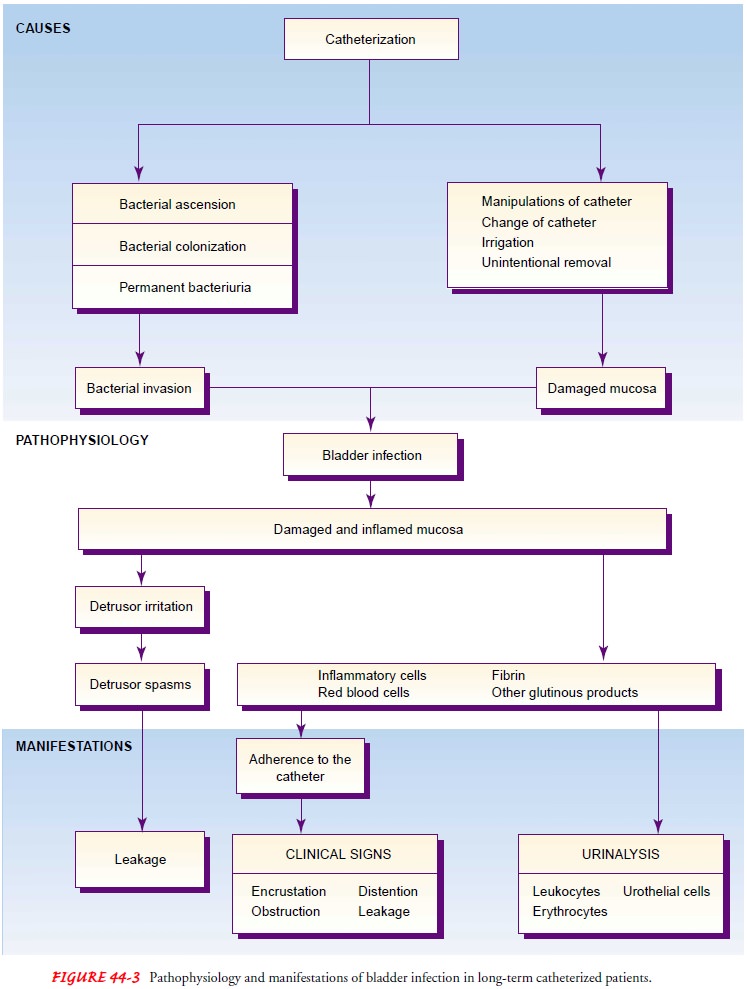
diagnosed. Figure 44-3 summarizes the sequence of events leading to infection
and leak-age of urine that often follow long-term use of an indwelling catheter
in elderly patients.
PREVENTING INFECTION
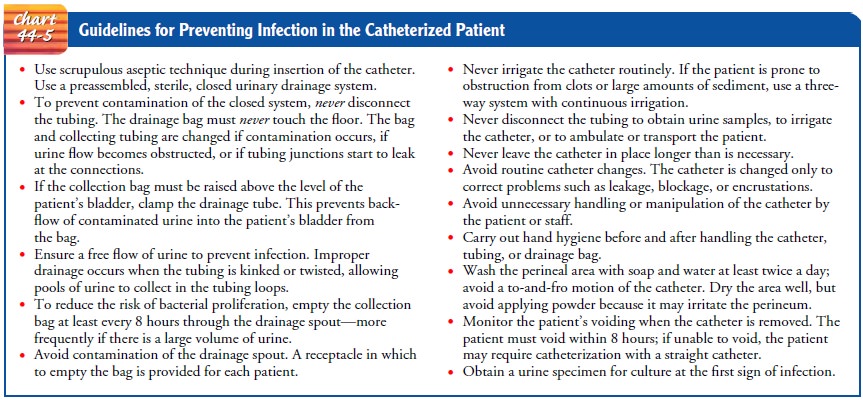
Certain
principles of care are essential to prevent infection in pa-tients with a
closed urinary drainage system (Chart 44-5). The catheter is a foreign body in
the urethra and produces a reaction in the urethral mucosa with some urethral
discharge. Vigorous cleaning of the meatus while the catheter is in place is
discour-aged, however, because the cleaning action can move the catheter to and
fro, increasing the risk of infection. To remove obvious en-crustations from
the external catheter surface, the area can be washed gently with soap during
the daily bath. The catheter is an-chored as securely as possible to prevent it
from moving in the urethra. Encrustations arising from urinary salts may serve
as a nucleus for stone formation; however, using silicone catheters results in
significantly less crust formation.
A
liberal fluid intake, within the limits of the patient’s cardiac and renal
reserve, and an increased urine output must be ensured to flush the catheter
and to dilute urinary substances that might form encrustations.
Urine
cultures are obtained as prescribed or indicated in mon-itoring the patient for
infection; many catheters have an aspiration (puncture) port from which a
specimen can be obtained.
Controversy
exists about the usefulness of taking cultures and treating bacteriuria in
patients who have symptoms of infection and who have indwelling catheters.
Bacteriuria is considered to be inevitable, and overtreatment may lead to
resistant strains of bacteria (Suchinski et al., 1999).
MINIMIZING TRAUMA
Trauma
to the urethra can be minimized by:
· Using an
appropriate-sized catheter
· Lubricating the catheter
adequately with a water-soluble lu-bricant during insertion
· Inserting the catheter
far enough into the bladder to prevent trauma to the urethral tissues when the
retention balloon of the catheter is inflated
Manipulation
of the catheter is the most common cause of trauma to the bladder mucosa in the
catheterized patient. In-fection then inevitably occurs when urine invades the
damaged mucosa.
The
catheter is secured properly to prevent it from moving, causing traction on the
urethra, or being unintentionally re-moved, and care is taken to ensure that
the catheter position per-mits leg movement. In male patients, the drainage
tube (not the catheter) is taped laterally to the thigh to prevent pressure on
the urethra at the penoscrotal junction, which can eventually lead to formation
of a urethrocutaneous fistula. In female patients, the drainage tubing attached
to the catheter is taped to the thigh to prevent tension and traction on the
bladder.
Care
is taken to ensure that any patient who is confused does not remove the
catheter with the retention balloon still inflated. This could cause bleeding
and considerable injury to the urethra (Phillips, 2000).
RETRAINING THE BLADDER
When
an indwelling urinary catheter is in place, the detrusor muscle does not
actively contract the bladder wall to stimulate emptying, because urine is
continuously draining from the blad-der. As a result, the detrusor may not
immediately respond to bladder filling when the catheter is removed, resulting
in either urine retention or urinary incontinence. This condition, known as
postcatheterization detrusor instability, can be managed with bladder
retraining (Chart 44-6).
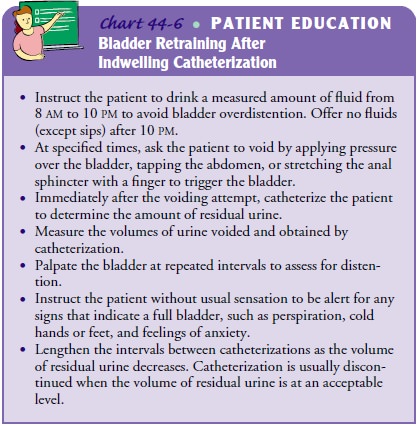
Immediately after the indwelling catheter is removed, the pa-tient is placed on a timed voiding schedule, usually every 2 to 3 hours. At the given time interval, the patient is instructed to void. The bladder is then scanned using a portable ultrasonic blad-der scanner. If 100 mL or more of urine remains in the bladder, straight catheterization may be performed for complete bladder emptying.
After a few days, as the nerve endings in the bladder wall become aware of
bladder filling and emptying, bladder func-tion usually returns to normal. If
the individual has had an in-dwelling catheter in place for an extended period,
bladder retraining will take much longer; in some cases, function may never
return to normal. If this occurs, long-term intermittent catheterization may
become necessary (Phillips, 2000).
ASSISTING WITH INTERMITTENT SELF-CATHETERIZATION
Intermittent self-catheterization provides periodic drainage of urine from the bladder. By promoting drainage and eliminating excessive residual urine, intermittent catheterization protects the kidneys, reduces the incidence of urinary tract infections, and improves continence.
It is the treatment of choice in patients with spinal cord injury
and other neurologic disorders, such as multi-ple sclerosis, when the ability
to empty the bladder is impaired. Self-catheterization promotes independence,
results in few com-plications, and enhances self-esteem and quality of life.
When
teaching the patient how to perform self-catheterization, the nurse must use
aseptic technique to minimize the risk of cross-contamination. The patient,
however, may use a “clean” (non-sterile) technique at home, where the risk of
cross-contamination is reduced. Either antibacterial liquid soap or
povidone-iodine (Betadine) solution is recommended for cleaning urinary
cath-eters at home. The catheter is thoroughly rinsed with tap water after
soaking in the cleaning solution. It must dry before reuse. It should be kept
in its own container, such as a plastic food-storage bag.
In
teaching the patient, the nurse emphasizes the importance of frequent
catheterization and emptying the bladder at the pre-scribed time. The average
daytime clean intermittent catheteri-zation schedule is every 4 to 6 hours and
just before bedtime. If the patient is awakened at night with an urge to void,
catheteri-zation may be performed after an attempt to void (Reilly, 2001).
The
female patient assumes a Fowler’s position and uses a mirror to help locate the
urinary meatus. She inserts the catheter 7.5 cm (3 in) into the urethra, in a
downward and backward di-rection. The male patient assumes a Fowler’s or
sitting position, lubricates the catheter and retracts the foreskin of the
penis with one hand while grasping the penis and holding it at a right angle to
the body. (This maneuver straightens the urethra and makes it easier to insert
the catheter.) He inserts the catheter 15 to 25 cm (6 to 10 in) until urine
begins to flow. After removal, the catheter is cleaned, rinsed, and wrapped in
a paper towel or placed in a plastic bag or case. Patients following this
routine should consult a primary health care provider at regular intervals to
assess urinary function and to detect complications.
If the
patient cannot perform intermittent self-catheterization, a family member may
be taught to carry out the procedure at reg-ular intervals during the day.
Another
self-catheterization option is creation of the Mitro-fanoff umbilical appendicovesicostomy,
which provides easy access to the bladder. In this procedure, the bladder neck
is closed and the appendix is used to gain access to the bladder from the skin
surface. A submucosal tunnel is created with the appendix; one end of the
appendix is brought to the skin surface and used as a stoma and the other end
is tunneled into the bladder. The ap-pendix may be used as an artificial
urinary sphincter when an alter-native is necessary to empty the bladder. In
children, the most common reason for the procedure is spina bifida. In adults,
a sur-gically prepared continent urine reservoir with a sphincter mecha-nism is
required in cases of bladder cancer, severe interstitial cystitis, or in males,
bladder exstrophy-epispadias complex when a radical cystectomy (surgical
removal of the bladder) is necessary. This pro-cedure for surgically creating a
sphincter, which is attached to an internal pouch reservoir that can be
catheterized, is possible only in individuals who have a healthy appendix
(Kajbafzadeh & Chubak, 2001; Uygur et al., 2001).
Related Topics