Chapter: Modern Medical Toxicology: Neurotoxic Poisons: Anaesthetics and Muscle Relaxants
Neuromuscular Blocking Agents
Neuromuscular Blocking Agents
Uses
·
Adjuvant in surgical anaesthesia to
obtain skeletal muscle relaxation.
·
Facilitation of orthopaedic
procedures such as correction of dislocations and alignment of fractures.
Facilitation of endotracheal intubation, laryngoscopy, bronchoscopy,
oesophagoscopy, etc.
·
Prevention of trauma during
electroconvulsive therapy.
Mode of Action
·
The essential mechanism of action of
all NMBs is inhibition of the effects of acetylcholine (ACH) on nicotinic
receptors at the neuromuscular junction (NMJ).
·
The depolarising NMBs (or DNMBs)
such as succinyl- choline (suxamethonium) produce muscle depolarisation in the
same way as ACH. The action of succinylcholine is prolonged because it is
relatively resistant to hydrolysis by true acetylcholinesterase.
·
The nondepolarising NMBs (or NDNMBs)
act by competi- tive inhibition of ACH at nicotinic receptors, and their action
is in general of shorter duration.
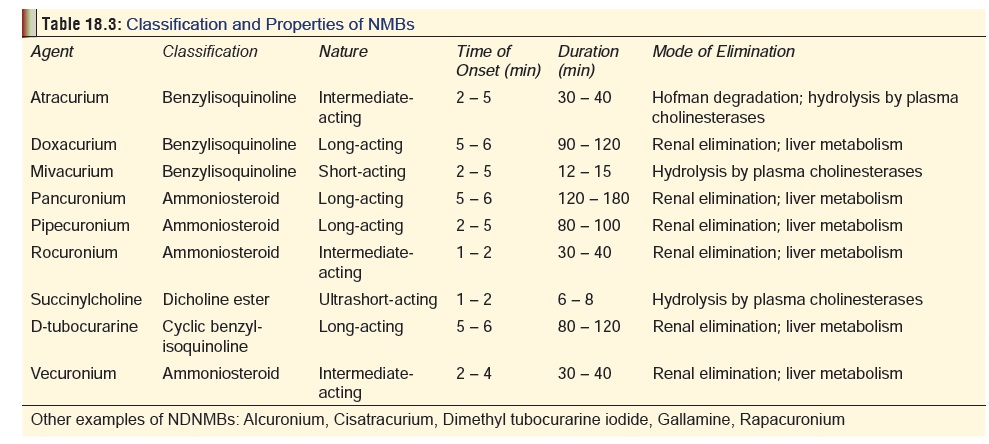
Toxicokinetics
·
The toxicokinetics of commonly used
NMBs is summarised in Table 18.3.
·
Succinylcholine is rapidly
hydrolysed by plasma pseudocho- linesterase to an intermediate metabolite
succinylmonocho- line. This metabolite is weaker in action than
succinylcholine, but because of its slower rate of hydrolysis may accumulate
and cause prolonged paralysis of the patient.
·
Succinylmonocholine is hydrolysed to
succinic acid and choline, neither of which has pharmacologic action.
·
Therapeutic doses produce the
following sequence of skel- etal muscle depression: heaviness to the eyelids,
difficulty in swallowing and talking, diplopia, progressive weakness of the
extremities, the neck, trunk, spine, intercostals, and diaphragm. The paralysis
recedes in the reverse.
Adverse Effects
· Prolonged apnoea and respiratory
paralysis.
· Rapacuronium has been voluntarily withdrawn from the market by the manufacturer, due to reports of an associa-tion with rapacuronium administration and the occurrence of bronchospastic events, including the occurrence of unexplained fatalities.
·
Cardiovascular collapse preceded by tachy- or bradycardia,
and hypo- or hypertension. Gallamine has a shorter dura- tion of action than
tubocurarine and, due to its blocking of the cardiac vagus, it may cause sinus
tachycardia and, occasionally, arrhythmias and hypertension.
·
Histamine-release effects: bronchospasm,
hypotension, excessive airway secretions.
·
Hyperkalaemia (with
succinylcholine): The potassium may originate from skeletal muscle, released by
depolarisation at the neuromuscular junction or from damaged muscle fibres
caused by incoordinate contractions The rise in potas- sium usually occurs 3 to
5 minutes after IV administration of succinylcholine, and is usually 0.5 to 1
mmol/L. The increase usually lasts less than 10 to 15 minutes.
·
Bradycardia may occur secondary to
severe hyperkalaemia and may progress rapidly to asystole or ventricular
fibril- lation in this setting. Use in patients with extensive burns, traumatic
muscle injury, paraplegia, hemiplegia, muscular dystrophy, multiple sclerosis,
prolonged pharmacologic neuromuscular blockade, upper motor neuron injury or
extensive denervation of skeletal muscle can predispose to severe hyperkalaemia
and ventricular arrhythmias.
·
Malignant hyperthermia: Malignant
hyperthermia (MH) is a rare, genetically influenced, potentially lethal
complica- tion associated with the use of inhalational anaesthetics,
amino-amide local anaesthetics, and some muscle relaxants (succinylcholine,
decamethonium, d-tubocurarine, and gallamine). It can also be precipitated in
susceptible indi- viduals by stress, hot environment, emotional excitement,
physical exertion, and infection. The genetic susceptibility to MH is due to a
mutation of the ryanodine receptor gene located in the region of 12–13.2 of
chromosome 19. This is responsible for decreased calcium uptake by the sarcoplasmic
reticulum of muscle cells leading to increase in myoplasmic calcium, which is
triggered by a nmber of agents. A number of aerobic and anaerobic metabolic
processes are set in motion resulting in excessive heat and CO2
and lactic acid production. Indications of MH during anaesthesia include the
following:
o Tachycardia
(unexplained).
o Tachypnoea,
cyanosis (unexplained).
o Rigidity
(masseters fail to relax for intubation).
o Marked
hyperthermia (late sign).
o Hypotension,
arrhythmias.
o Metabolic
acidosis.
o Hyperkalaemia,
hypercalcaemia.
o Electrolyte
disturbances.
o Rhabdomyolysis,
disseminated intravascular coagula- tion (DIC), renal failure.
o Pulmonary
oedema.
Early diagnosis can be aided by arterial blood gas analysis
(hypoxaemia), electrolyte level estimation, oximetry, and end-tidal CO2
measurement (increased). Death in MH may be due to ventricular fibrillation,
DIC, renal failure, cerebral oedema, or pulmonary oedema.
·
Succinylcholine-induced
rhabdomyolysis from prolonged fasciculations or malignant hyperthermia can lead
to renal failure. Elevated serum levels of creatine phosphokinase (CPK) and
myoglobin commonly follow IV administration of succinylcholine.![]()
·
Persistent weakness (especially in
critically ill patients subjected to prolonged ventilation) referred to as ICUneuromuscular syndrome. Recovery may
take upto 6months. Precautionary measures are necessary to minimise the
possibility of this distressing complication.
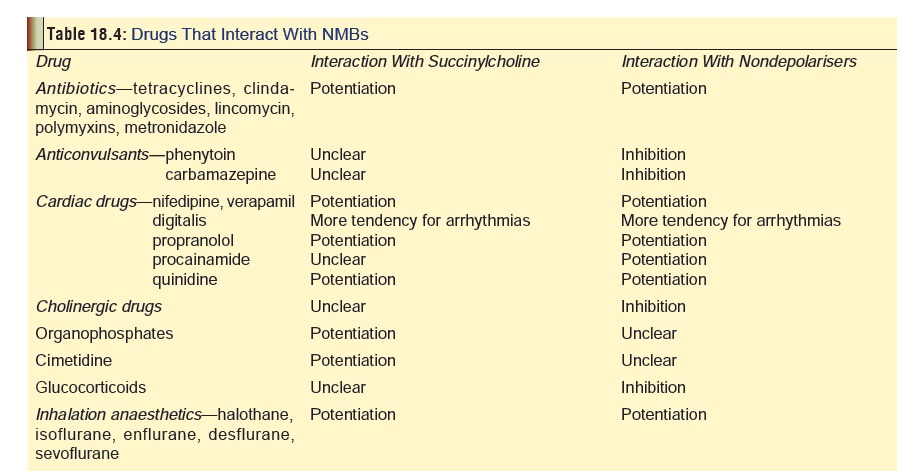
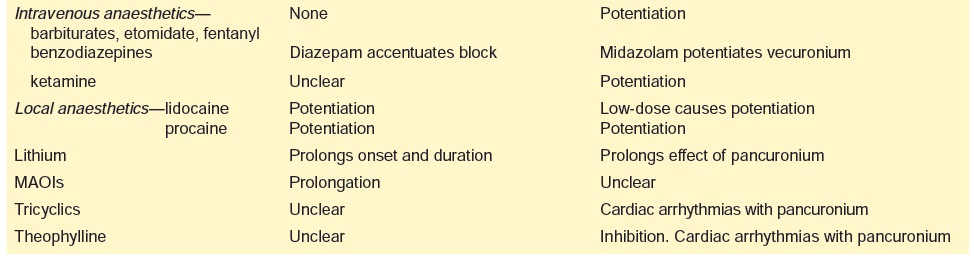
Drug Interactions
·
Some of the important drug
interactions with NMBs are listed in Table
18.4.
Clinical (Toxic) Features
· Succinylcholine (succinyldicholine,
diacetylcholine, or suxamethonium) is a bis-quaternary ammonium ion composed of
two acetylcholine molecules connected by their acetate groups. The dose
necessary to produce neuromuscular blockade and respiratory paralysis in adults
ranges from 0.3 to 1.1 mg/kg in adults (mean 0.6 mg/kg). Succinylcholine use is
sometimes associated with prolonged apnoea which may be due to genetically
determined atypical pseudocholinesterase (incidence 1 : 2500), or due to
exposure to cholinesterase inhibitors such as organophosphates.
o
Adverse effects of succinylcholine include cardiac
arrhythmias, hyperkalaemia, increased intracranial pressure, increased
intraocular pressure, increased intra-gastric pressure, myalgia, muscle
fasciculation, muscle rigidity (especially masseters), malignant hyperthermia,
rhabdomyolysis and myoglobinuria.
o
In children with unsuspected myopathies (especially
Duchenne’s muscular dystrophy), acute rhabdomyol-ysis, severe
hyperkalaemia, and cardiac arrest can occur, and hence it is advisable not to
use succinylcholine in the paediatric age group (particularly boys under the
age of 8 years) except for emergency intubation.
o
Succinylcholine is also well known for causing anaphy-laxis
in susceptible individuals (mostly women) which manifests as rapid circulatory
collapse without other conventional signs such as skin rash or wheezing.
· Tubocurarine and all other
curariform blocking agents are derived from curare (Fig 18.3), a large vine, found in the canopy of the South American
rainforest. Overdose causes complete skeletal muscle paralysis without
affecting consciousness. Initially the small muscles of the eyes, ears,
fingers, and toes are paralysed, followed by face and neck, upper and lower
limbs, and finally the diaphragm and inter-costal muscles, leading to
respiratory failure.

o Metocurine produced by methylation of tubocurarine is twice as potent while doxacurium is a long-acting NMB without histamine-releasing effects. Unlike the others, it is metabolised rapidly at first to laudanosine, and later to an acrylate moiety both of which do not possess NMB property.
o
cis-Atracurium is a purified atracurium isomer which is much
more potent, and unlike its parent compound is not associated with histamine
release.
o
Mivacurium is a short-acting drug composed of a mixture of 3
stereo-isomers, but may sometimes cause prolonged block.
o
Pancuronium is a synthetic bis-quaternary aminosteroid which
has a selective cardiac antimuscarinic (atropine-like) action resulting in
increased heart rate and blood pressure. It is partly metabolised and undergoes
some degree of deacetylation in the liver, which is responsible for prolonged
effects in the presence of hepatic insuf-ficiency.
o
Vecuronium is a derivative of pancuronium with similar
potency, but is less prone to induce tachycardia and hypertension.
o
Pipecuronium is a long-acting analogue producing a block of
long duration.
o
Rocuronium is known for its rapid onset of action and does
not produce histamine release or significant cardiac effects.
Treatment
·
Reversal of NDNMB block can be achieved by
anti-cholinesterases such as neostigmine (0.040–0.080 mg/ kg), pyridostigmine
(0.2–0.4 mg/kg), or edrophonium (0.5–1.0 mg/kg), in combination with
antimuscarinic agents such as glycopyrrolate (0.01–0.02 mg/kg) or atropine
(0.02–0.03 mg/kg).
·
Overdose with depolarising agents such as succinylcho-line
cannot be reversed pharmacologically, and must be managed with prolonged
assisted ventilation.
·
Physostigmine, neostigmine and other anticholinesterase
drugs, including edrophonium, are contraindicated as antidotes to
succinylcholine because they actually prolong its action by interfering with
metabolism by cholinesterase. Determine pseudocholinesterase activity in
patients with unexpectedly prolonged effects. However, many of the patients who
react abnormally to succinylcholine have qualitative rather than quantitative
defects in plasma pseu-docholinesterase.
·
Maintain patent airway and supply 100% oxygen. Assisted
ventilation is usually required. Most patients will recover if
adequate airway, ventilation and oxygen-ation are established rapidly.
Treatment
of malignant hyperthermia:
o
Discontinue all triggering agents.
o
Hyperventilate with 100% oxygen (10 L/min).
o
Give dantrolene sodium 2 to 3 mg/kg IV bolus, followed by
increments upto a maximum of 10 mg/kg. Stop dantrolene when the signs of MH are
controlled, and administer it subsequently at 1 mg/ kg IV 6th hourly for 1 to 2
days, and then the same dose orally for 1 more day.
o
Give sodium bicarbonate to correct metabolic acidosis (1 to
2 mEq/kg).
o
Treat hyperthermia with
IV
iced saline, 15 ml/kg, q15 min × 3.
Lavage
of stomach, bladder and rectum with iced saline.
Skin
surface cooling with ice.
o
Treat persistent arrhythmias with standard anti-arrhythmic
drugs (except calcium channel blockers which can cause or aggravate
hyperkalaemia).
o
Treat hyperkalaemia with hyperventilation, sodium
bicarbonate, IV glucose, and insulin. Dangerous hyperkalaemia may necessitate
calcium administra-tion (2 to 5 mg/kg of calcium chloride).
o
Ensure adequate urine output (more than 2 ml/kg/hr).
o
Monitor
End-tidal
CO2.
Arterial
and venous blood gases.
Serum
potassium and calcium.
Clotting
studies.
Urine
output.
· Treat cardiovascular failure in the
usual way.
· Treat severe hyperkalaemia
(associated arrhythmias, QRS widening) aggressively. Monitor ECG continuously
during and after therapy.
o
Calcium chloride: Adult: 5 ml IV bolus of a 10%
solu-tion over 5 minutes; Child: 0.2 to 0.3 ml/kg of a 10% solution over 5 to
10 minutes (20 to 30 ml/kg /dose).
o
Sodium bicarbonate: Adult or Child: 1–2 mEq/kg IVbolus.
o
Insulin/dextrose: Adult: 5 to 10 units regular
insulinIV bolus with 100 ml of D50 IV immediately; monitor serum glucose every
30 minutes; Child: 0.5 to 1 gm/ kg dextrose as D25 or D10 IV followed by 1 unit
of regular insulin for every 4 grams of dextrose infused; monitor serum glucose
every 30 minutes.
o
Sodium polystyrene sulfonate: Adult 15 to 60 grams by nasogastric tube or rectal enema;
Child: 1 gm/kg by nasogastric tube or rectal enema.
·
Pretreatment with 0.125 mg/kg IV succinylcholine followed in
60 seconds by 1 mg/kg IV may reduce postoperative muscle fasciculations and
pain in adults. Pretreatment with d-tubocurarine (0.05 mg/kg) may decrease
myoglobinaemia.
·
For rhabdomyolysis: Early aggressive fluid replacement is
the mainstay of therapy and may help prevent renal insufficiency. Diuretics
such as mannitol or furosemide may be needed to maintain urine output. Urinary
alka-linisation is NOT routinely recommended.
·
In susceptible patients, succinylcholine can produce a rise
in ICP that may lead to herniation. Pretreatment with a low dose of a
nondepolarising agent such as pancuronium 0.01 mg/kg IV or low dose
succinylcholine 0.1 mg/kg IV 3 to 5 minutes prior to administration of full
dose succinylcholine, may blunt the rise in ICP.
·
In patients with renal failure, haemodialysis may be
effec-tive in reversing prolonged neuromuscular blockade due to tubocurarine or
pancuronium. However, dialysis will not be effective for overdose of atracurium
or vecuronium since these agents are not renally excreted.![]()
Related Topics