Chapter: Clinical Anesthesiology: Anesthetic Management: Anesthesia for Cardiovascular Surgery
Myocardial Preservation
MYOCARDIAL PRESERVATION
Optimal results in cardiac surgery
require an expe-ditious and complete surgical repair with minimal physical
trauma to the heart. Meanwhile, several techniques are used to prevent
myocardial damage and maintain normal cellular integrity and function during
CPB. Nearly all patients sustain at least mini-mal myocardial injury during
cardiac surgery. With good preservation techniques, however, most of the injury
is reversible. Although myocardial injury can be related to the hemodynamic
instability or sur-gical technique, it most commonly appears to be related to
incomplete myocardial preservation dur-ing CPB. Injury related to hemodynamic
instability results from an imbalance between oxygen demand and supply,
producing cell ischemia. After ischemia, reperfusion injury may also play a
role. Reperfusion following a period of ischemia may produce excess
oxygen-derived free radicals, intracellular calcium overload, abnormal
endothelial–leukocyte interac-tions, and myocardial cellular edema. Patients at
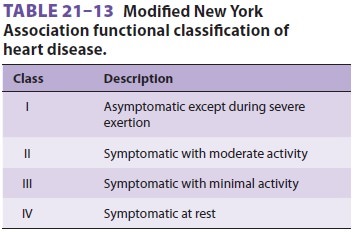
greatest risk are those with poor ventricular func-tion (as measured
preoperatively) (see Table 21–13) those with ventricular hypertrophy, and those
with diffuse severe coronary artery disease. Inadequate myocardial preservation
is usually manifested at the end of bypass as a persistently reduced cardiac
output, worsened ventricular function by TEE, or cardiac arrhythmias.
Electrocardiographic signs of myocardial ischemia are often difficult to detect
due to frequent use of electrical pacing. Myocardial “stunning,” resulting from
ischemia and reperfu-sion, produces systolic and diastolic dysfunction that is
reversible with time. The stunned myocar-dium usually responds to positive
inotropic drugs. Myocardial necrosis, on the other hand, produces irreversible
injury.
Aortic cross-clamping during CPB
completely excludes the coronary arteries from the general-ized bypass machine
flow to the body, reducing coronary blood flow to 0. Although it is difficult
to estimate a safe period for cross-clamping because of differing
vulnerabilities among patients and dif-fering techniques for myocardial
preservation, CPB times longer than 120 min (while often unavoid-able) increase
risk relative to shorter bypass times. Myocardial ischemia during bypass may
occur not only during aortic clamping, but also after release of the cross-clamp.
Low arterial pressures, coro-nary embolism (from thrombi, platelets, air, fat,
or atheromatous debris), reperfusion injury, coronary artery or bypass graft
vasospasm, and contortion of the heart—causing compression or distortion of the
coronary vessels—are all possible causes. Areas of the myocardium distal to a
high-grade coronary obstruction are at greatest risk.
Ischemia causes depletion of high-energy
phos-phate compounds and an accumulation of intracel-lular calcium. When
coronary blood flow ceases, creatine phosphate and anaerobic metabolism become
the principal sources of cellular energy; fatty acid oxidation is impaired.
Unfortunately, these energy stores rapidly become depleted, and the
pro-gressive acidosis that develops limits glycolysis.
Cardioplegic solutions maintain normal
cellu-lar integrity and function during CPB by reducing energy expenditure and
preserving the availability of high-energy phosphate compounds. Although
measures directed at increasing or replenishing energy substrates in the form
of glucose or gluta-mate/aspartate infusions are used, the emphasis of
myocardial preservation has been on reducing cel-lular energy requirements to
minimal levels. This is accomplished initially by the use of potassium
cardioplegia (below). The initial dose of cardiople-gic solution may be
hypothermic or may start warm (“hot shot”) and progress to cold. Maintenance of
myocardial protection may be facilitated by sys-temic and topical cardiac
hypothermia (ice slush). Myocardial hypothermia reduces basal metabolic oxygen
consumption, and potassium cardioplegia minimizes energy expenditure by
arresting both electrical and mechanical activity. Myocardial temperature is
often monitored directly; 10–15°C is usually
considered desirable. Cardioplegic solu-tions can be administered either
antegrade through a catheter placed in the proximal aorta between the aortic
clamp and the aortic valve, or retrograde through a catheter placed through the
right atrium into the coronary sinus.
Ventricular fibrillation and distention
(previ-ously discussed) are important causes of myocardial damage. Ventricular
fibrillation can dangerously increase myocardial oxygen demand, whereas
dis-tention not only increases oxygen demand but also reduces oxygen supply by
interfering with suben-docardial blood flow. The combination of the two is
particularly bad. Other factors that might contribute to perioperative
myocardial damage include the use of excessive doses of positive inotropes or
calcium salts. In open heart procedures, de-airing of cardiac chambers and
venting before and during initial car-diac ejection are critically important in
preventing cerebral or coronary air embolism (and strokes). Removing air from
coronary grafts during bypass procedures is similarly important.
Depending on the amount and the location
of coro-nary emboli, even small air bubbles can cause vary-ing degrees of
ventricular dysfunction at the end of CPB. To some extent, air emboli may
preferentially find their way into the right (versus left) coronary ostium
because of its superior location on the aortic root in the supine patient.
Potassium Cardioplegia
The most widely used method of arresting
myo-cardial electrical activity is the administration of potassium-rich
crystalloid or blood–crystalloid solu-tions. Following initiation of CPB and
aortic cross-clamping, the coronary circulation is perfused inter-mittently
with (usually cold) cardioplegic solutions. The resulting increase in
extracellular potassium concentration reduces the transmembrane potential.
Eventually, the heart is arrested in diastole. Usually, cold cardioplegia must
be repeated at intervals (about every 30 min) because of gradual washout and
rewarming of the myocardium. The heart is sub-ject to warming by contact with
blood in the adja-cent descending aorta and by contact with warmer ambient air
in the surgical theater. Moreover, mul-tiple doses of cardioplegia solutions
may improve myocardial preservation by preventing an excessive accumulation of
metabolites that inhibit anaerobic metabolism.
Although the exact recipe varies from
center to center, the essential ingredient of the induction dose of
cardioplegic solution is the same: an elevated potassium (10–40 mEq/L)
concentration. Potassium concentration is kept below 40 mEq/L, because higher
levels can be associated with an excessive potassium load and excessive
potassium concentra-tions at the end of termination of bypass perfusion. Sodium
concentration in cardioplegic solutions is usually less than in plasma (<140 mEq/L) because ischemia tends to increase
intracellular sodium con-tent. A small amount of calcium (0.7–1.2 mmol/L) is
needed to maintain cellular integrity, whereas magnesium (1.5–15 mmol/L) is
usually added to control excessive intracellular influxes of calcium. A
buffer—most commonly bicarbonate—is necessary to prevent excessive buildup of
acid metabolites; in fact, alkalotic perfusates are reported to produce better
myocardial preservation. Alternative buffers include histidine and tromethamine
(also known as THAM). Other components may include hypertonic agents to control
cellular edema (mannitol) and agents thought to have membrane-stabilizing
effects (lidocaine or glucocorticoids). Energy substrates are provided as
glucose, glutamate, or aspartate. The question of whether to use crystalloid or
blood as a vehicle for achieving cardioplegia remains con-troversial, although
blood cardioplegia has become very common in North America. Evidence suggests
that at least some groups of high-risk patients may do better with blood cardioplegia.
Certainly, oxy-genated blood cardioplegia may contain more oxy-gen than
crystalloid cardioplegia.
Because cardioplegia may not reach areas
distal to high-grade coronary obstructions (the areas that need it most), many
surgeons administer retrograde cardioplegia through a coronary sinus catheter.
Some centers have reported that the combination of antegrade plus retrograde
cardioplegia is superior to either technique alone. Others have suggested that
continuous warm blood cardioplegia is supe-rior to intermittent hypothermic
cardioplegia for myocardial preservation, but many surgeons avoid continuous
cardioplegia so that they can operate in a “bloodless” surgical field.
Moreover, warm cardiac surgery raises additional concerns about loss of the potentially
protective effects of systemic hypother-mia against cerebral injury, when true
normother-mia (rather than tepid bypass) is maintained.
As discussed previously, with prolonged
myocar-dial ischemic times (cross-clamp time), reperfusion of the myocardium
can lead to extensive cell injury, rapid accumulation of intracellular calcium,
and potentially irreversible cellular necrosis. This process has long been
attributed to depletion of endogenous free radical scavengers during CPB and
accumulation of deleterious oxygen-derived free radicals. Free radi-cal
scavengers, such as mannitol, may help decrease reperfusion injury and are
typical constituents of cardioplegic solutions and bypass “priming” solu-tions.
Several steps may help limit reperfusion injury before unclamping of the aorta.
Just prior to reperfu-sion, the heart may be perfused by a reduced potas-sium
cardioplegic solution that serves to wash out accumulated metabolic byproducts.
Alternatively, a “hot shot” or warm blood cardioplegic solution may be
administered to wash out byproducts and replen-ish metabolic substrates.
Hypercalcemia should be avoided in the immediate reperfusion period.
Reperfusion pressures should be controlled closely because of altered coronary
autoregulation. Systemic perfusion pressure is reduced just prior to clamp
release; it is then brought up initially to about 40 mm Hg before gradually
being increased and maintained at about 70 mm Hg. To further minimize metabolic
requirement, the heart should have the opportunity to recover and resume
contracting in an empty state for some additional time (5–10 min), and acidosis
and hypoxia should be corrected before attempting to wean the patient from
bypass perfusion.
Inadequate myocardial protection or
inade-quate washout and recovery from cardioplegia can result in asystole,
atrioventricular conduction block, or a poorly contracting heart at the end of
bypass. Excessive volumes of hyperkalemic cardioplegic solutions may produce
persisting systemic hyper-kalemia. Although calcium salt administration
par-tially offsets hyperkalemia, excessive calcium can promote and enhance
myocardial damage. In the usual patient myocardial performance improves with
time as the contents of the cardioplegia are cleared from the heart.
Related Topics