Chapter: Clinical Anesthesiology: Anesthetic Management: Anesthesia for Cardiovascular Surgery
Anesthetic Management of Cardiac Surgery: Bypass Period
Bypass Period
Initiation
Once the cannulas are properly placed
and secured, the ACT is acceptable, and the perfusionist is ready, CPB is
initiated. The clamps placed across the venous cannula(s) during insertion are
removed, and the main CPB pump is started. Establishing the adequacy of venous
return to the pump reservoir is critical. Normally, the reservoir level rises
and CPB pump flow is gradually increased. If venous return is poor, as shown by
a decreasing reservoir level, the pump prime will quickly empty and air can
enter the pump circuit. When the venous reservoir falls the cannulas should be
checked for proper place-ment and for forgotten clamps, kinks, or an air lock.
Under these circumstances, pump flow should be slowed until the problem is resolved.
Adding volume (blood or colloid) to the reservoir may be necessary. With full
CPB and unimpeded venous drainage, the heart should empty; failure to empty or
progressive distention implies malpositioning of the venous cannula or aortic
regurgitation. In the rare case of severe aortic insufficiency that limits the
extent of peripheral perfusion, immediate aortic cross-clamp-ing (and
cardioplegia) may be necessary.
Flow & Pressure
Systemic mean arterial pressure is
closely moni-tored as pump flow is gradually increased to 2– 2.5 L/min/m2. At the onset of CPB, systemic arte-rial pressure
usually decreases abruptly. Initial mean systemic arterial (radial) pressures
of 30–40 mm Hg are not unusual. This decrease is usually attributed to abrupt
hemodilution, which reduces blood vis-cosity and effectively lowers SVR. It is
often treated with increased flow and vasopressors.
Persistent and excessive decreases (<30
mm Hg) should prompt a search for unrecognized aor-tic dissection. If
dissection is present, CPB must be temporarily stopped until a cannula can be
placed distally in the “true” aortic lumen. Other possible causes for
hypotension include inadequate pump flow from poor venous return or a pump
malfunc-tion, or pressure transducer error. Factitious hyper-tension has been
reported when the right radial artery is used for monitoring and the aortic
cannula is directed toward the innominate artery.
The relationship between pump flow, SVR,
and mean systemic arterial blood pressure may be con-ceptualized as follows:
Mean arterial pressure = Pump flow × SVR
Consequently, with a constant SVR, mean
arte-rial pressure is proportional to pump flow. Similarly, at any given pump
flow, mean arterial pressure is pro-portional to SVR. To maintain both adequate
arte-rial pressures and blood flows one can manipulate pump flow and SVR. Most
centers strive for blood flows of 2–2.5 L/min/m2
(50–60 mL/kg/min) and mean arterial pressures between 50 and 80 mm Hg.
Metabolic flow requirements generally decline with decreasing core body
temperature. Evidence also suggests that during deep hypothermia (20–25°C), mean blood
pressures as low as 30 mm Hg may still be consistent with adequate cerebral
blood flow and cerebral oxygen delivery. SVR can be increased with
phenylephrine, vasopressin, or norepinephrine.
Increased systemic arterial pressures (>150
mm Hg) are deleterious and may promote aortic dissec-tion or cerebral
hemorrhage. Generally, when mean arterial pressure exceeds 100 mm Hg,
hypertension is said to exist and is treated by decreasing pump flow or
increasing the concentration of a volatile agent to the oxygenator inflow gas.
In the rare instance that the hypertension is refractory to these maneuvers or
if pump flow is already low, a vasodilator such as clevidipine, nicardipine, or
nitroprusside is used.
Monitoring
Additional monitoring during CPB
includes the pump flow rate, venous reservoir level, arterial inflow line
pressure (see above), blood (perfusate and venous) and myocardial temperatures,
and in-line (arterial and venous) oxygen saturations. In-line pH, CO2 tension, and oxygen tension sensors are also
available. Blood gas tensions and pH should be
confirmed by direct measurements . In
the absence of hypoxemia, low venous oxygen satu-rations (<70%), a progressive metabolic acidosis, or reduced
urinary output may indicate inadequate flow rates.
During bypass, arterial inflow line
pressure is almost always greater than the systemic arterial pressure recorded
from a radial artery or even an aortic catheter. The difference in pressure
represents the pressure drop across the arterial filter, the arte-rial tubing,
and the narrow opening of the aortic cannula. Nonetheless, monitoring this
pressure is important in detecting problems with an arterial inflow line.
Inflow pressures should remain below 300 mm Hg; higher pressures may indicate a
clogged arterial filter, obstruction of the arterial tubing or cannula, or
aortic dissection.
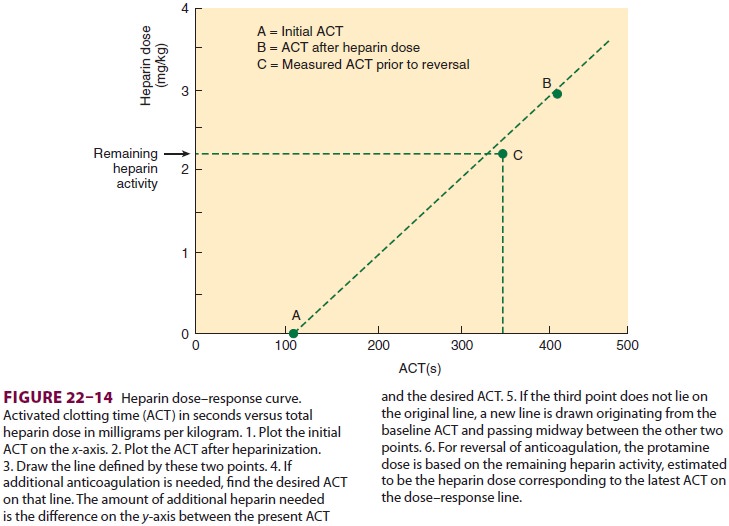
Serial ACT, hematocrit, and potassium
mea-surements are performed during CPB. Blood glucose should be checked even in
patients with-out a history of diabetes. The ACT is measured immediately after
bypass and then every 20–30 min thereafter. Cooling generally increases the
half-life of heparin and prolongs its effect. Some centers calculate a heparin
dose–response curve to guide calculation of heparin dosing and protamine
rever-sal (Figure
22–14). The hematocrit is usually not allowed fall much below
20–25%. Red cell trans-fusions into the pump reservoir may be necessary. Marked
increases in serum potassium concentra-tions (secondary to cardioplegia) are
usually treated with a furosemide-induced diuresis.
Hypothermia & Cardioplegia
Moderate (26–32°C) or deep (20–25°C) hypother-mia is used routinely for many
procedures. The lower the temperature, the longer the time required for cooling
and rewarming. Low temperatures, however, permit lower CPB flows to be used
safely. At a temperature of 20°C, flows as low as 1.2 L/min/ m2 may be adequate.
Hypothermia produces characteristic
changes in the ECG including the Osborne wave, a charac-teristic positive
deflection between the QRS and ST segments. Ventricular fibrillation often
occurs as the heart is cooled below 28–29°C. Cardioplegia should be established
immediately, as fibrillation consumes high-energy phosphates at a greater rate
than slower rhythms. Cardioplegia is achieved by cross-clamping the ascending
aorta proximal to the aortic inflow can-nula and (as previously described)
infusing cardio-plegia solution through a small catheter proximal to the
cross-clamp or directly into the coronary ostia if the aorta is opened (eg, for
aortic valve replacement). Many surgeons routinely employ retrograde
cardio-plegia via a catheter in the coronary sinus (see above). During
aortocoronary bypass grafting, cardioplegia solution may also be given through
the graft when the surgeon elects to perform the distal anastomosis first.
Ventilation
Ventilation of the lungs is discontinued
when ade-quate pump flows are reached and the heart stops ejecting blood.
Following institution of full CPB, ventricular ejection continues briefly until
the left ventricular volume reaches a critically low level. Discontinuing
ventilation prematurely when there is any remaining pulmonary blood flow acts
as a right-to-left shunt that can promote hypoxemia. The importance of this
mechanism depends on the relative ratio of remaining pulmonary blood flow to
pump flow. At some centers, once ventilation is stopped, oxygen flow is
continued in the anesthesia circuit with a small amount of continuous positive
airway pressure (5 cm H2O) in the hope of prevent-ing postoperative
pulmonary dysfunction. Most centers either stop all gas flow or continue a low flow of oxygen (1–2 L/min) in
the anesthesia circuit. Ventilation is resumed at the conclusion of CPB in
anticipation of the heart beginning to eject blood.
Management of Respiratory Gases
Th
ere formerly was controversy about whether to use temperature-corrected (pH
stat) or uncor-rected (α-stat) arterial blood gas tensions during hypothermic
CPB in adults. The controversy stemmed from the fact that the solubility of a
gas increases and the neutral pH (ie, the pH at which concentrations of H+ and
OH − ions are the same) of water increases with hypothermia. As a result of the
former effect, although total CO2 content does not change (in a closed system),
the partial pressure of CO2 will decrease as blood temperature drops. The
problem is most significant for arterial CO2 ten-sion because of its effect on
arterial pH and cerebral blood flow. As the temperature decreases, the plasma
bicarbonate concentration does not change, but the decrease in arterial CO 2
tension tends to increase pH and make blood alkalotic (by normothermic
defini-tions). Blood with a CO 2 tension of 40 mm Hg and a pH of 7.40 at 37°C,
when cooled to 25°C, will have a CO2 tension of about 23 mm Hg and a pH of
7.60.
Normally—regardless
of the patient’s tempera-ture—blood samples are heated to 37°C
in blood gas analyzers before gas tensions are measured. If a temperature-corrected
reading is desired, a table or a program in the blood gas machine can be used
to estimate what would be the gas tension and pH if they had been measured at
the patient’s temperature. The practice of temperature correcting gas tensions
with the goal of maintaining a constant CO 2 ten-sion of 40 mm Hg
and a constant pH of 7.40 during hypothermia is referred to as pH-stat management. During hypothermic
CPB, pH-stat management, which may require adding CO2 to the
oxygenator gas inflow, increases total blood CO2 content. Under
these conditions, cerebral blood flow increases (due to increased CO2
tension relative to α-stat manage-ment) more than is
required based on oxygen con-sumption. Increased cerebral blood flow is useful
to increase uniformity of brain cooling prior to deep hypothermic circulatory
arrest (more often used in children than adults). On the other hand, increased
cerebral blood flow can also direct a greater frac-tion of atheromatous
arterial emboli to the brain— a greater concern than uniformity of brain
cooling during cardiac surgery in adults.
The
use of uncorrected gas tensions during hypothermia—α-stat management—is
the rule in adults and is common in children when circulatory arrest will not
be used. The basis of this approach is that preservation of normal protein
function depends on maintaining a constant state of intra-cellular
electroneutrality (the balance of charges on proteins). At physiological pH,
these charges are primarily located on the imidazole rings of histi-dine
residues (referred to as α residues). Moreover, as temperature decreases,
Kw—the dissociation constant for water—also decreases (p Kw increases).
Therefore, at lower temperatures, the electroneutral-ity of aqueous solutions,
where [H +] = [OH−], corre-sponds to a lower [H+] (a higher pH). Hypothermic
“alkalosis” thus does not necessarily reflect [OH–] > [H+] but rather an absolute
decrease in both [H +] and [OH–]. Hypothermic CPB with α-stat management does
not require addition of CO 2 to the oxygenator: the total CO2 content of blood
and the electroneu-trality are unchanged. In contrast to pH-stat man-agement,
α-stat management appears to preserve cerebral autoregulation of blood flow.
Despite the theoretical and observed differences, in most stud-ies comparisons
between the two techniques fail to reveal appreciable differences in patient
outcomes except in children undergoing circulatory arrest.
Anesthesia
Hypothermia
(<34°C)
potentiates general anesthetic
potency, but failure to give anes-thetic agents, particularly during rewarming
on CPB, may result in awareness and recall. With light anesthesia hypertension
may be seen and, if muscle paralysis is also allowed to wear off, the patient
may move. Consequently, additional doses of anesthetic agents may be necessary
during CPB. Reduced con-centrations of a volatile agent (eg, 0.5–0.75%
isoflu-rane) via the oxygenator are frequently used. The volatile agent
concentration may need to be reduced to a value that does not depress
contractility imme-diately prior to termination of bypass if residual
myocardial depression is apparent. Those relying on opioids and benzodiazepines
for anesthesia dur-ing CPB may need to administer additional doses of these
agents or commence a propofol infusion. Some clinicians routinely administer a
benzodiazepine (eg, midazolam) or scopolamine (0.2–0.4 mg) when rewarming is
initiated. Alternatively, a propofol, opi-oid, or ketamine–midazolam infusion
may be con-tinued throughout CPB. Sweating during rewarming is common and
usually indicates a hypothalamicresponse to perfusion with warm blood (rather
than “light” anesthesia). During rewarming, blood temperature should not exceed
core temperature by more than 2°C.
Cerebral Protection
The incidence of neurobehavioral
deficits after CPB varies widely, depending on how long after surgery the
examination is performed and the cri-teria for diagnosis. In the first week
after surgery the incidence may be as high as 80%. Fortunately, in most
instances, these deficits are transient. Neurobehavioral deficits detectable 8
weeks or more (20–25%) after operation or strokes (2–6%) are less common.
Factors that have been associated with neurological sequelae include increased
numbers of cerebral emboli, combined intracardiac (valvular) and coronary
procedures, advanced age, and preex-isting cerebrovascular disease.
During open-heart procedures, deairing
of car-diac chambers, assumption of a head-down position, and venting before
and during initial cardiac ejection are important in preventing gas emboli.
Some centers fill the surgical field with CO2,
a gas that if entrained and embolized will more rapidly be reabsorbed. TEE can
detect residual air within the heart and the need for further deairing
procedures. During coronary bypass procedures, minimizing the amount of aortic
manipulation, the number of aortic clampings, and the number of graft sites on
the surface of the aorta, and using sutureless proximal anastomotic devices may
help reduce atheromatous emboli. Palpation of the aorta, TEE, and especially
epiaortic echocar-diography can help identify high-risk patients and guide
management. Epiaortic echocardiography is the most sensitive and specific
technique.
Although embolic phenomena appear
respon-sible for most neurological deficits, the contribu-tion of cerebral
hypoperfusion remains unclear. The data are controversial and sparse that
prophy-lactic drug infusions (eg, barbiturates or propofol to suppress
electroencephalographic activity) imme-diately before and during intracardiac
(open ven-tricle) procedures will decrease the incidence and severity of
neurological deficits. Prior to circulatory arrest with very deep hypothermia,
some clinicians administer a corticosteroid (methylprednisolone,30 mg/kg, or
the equivalent dose of dexametha-sone) and mannitol (0.5 g/kg). The head is
also covered with ice bags (avoiding the eyes). Surface cooling delays
rewarming and may also facilitate adequacy of brain cooling. A long list of
drugs has been tested and has failed to improve cerebral out-comes after heart
surgery. Human studies during cardiac surgery have not shown improved
neu-robehavioral outcomes with prophylactic adminis-tration of calcium channel
blockers (nimodipine), N-methyl-d-aspartat-e
(NMDA) antagonists (rema-cemide), free radical scavengers (pegorgotein),
sedative-hypnotics (thiopental, propofol, or clome-thiazole), or lazaroids
(tirilazad).
Related Topics