Chapter: Clinical Anesthesiology: Anesthetic Management: Anesthesia for Cardiovascular Surgery
Anesthetic Management of Cardiac Surgery: Postbypass Period
Postbypass Period
Following CPB, bleeding is controlled,
bypass can-nulas are removed, anticoagulation is reversed, and the chest is
closed. Systolic arterial pressure is generally maintained at less than 140 mm
Hg to minimize bleeding. Checking for bleeding, par-ticularly from the
posterior surface of the heart, requires lifting the heart, which can cause
periods of precipitous hypotension. Some surgeons will need to be informed of
the extent and duration of the hypotension; others have greater situational
awareness. The atrial cannula(s) is removed before the aortic cannula in case
the latter must be used to rapidly administer volume to the patient. Most
patients need additional blood volume after ter-mination of bypass.
Administration of blood, col-loids, and crystalloid is guided by filling
pressures (and observation of the left ventricle on TEE), and the postbypass
hematocrit. A final hematocrit of 25–30% is desirable, but is not mandatory.
Blood remaining in the CPB reservoir can be transfused via the aortic cannula
(while it remains in place) or washed and processed by a cell-saver device and
given intravenously. Frequent ventricular ectopy may reflect electrolyte
disturbances or residual ischemia and should be treated with amiodarone (or
lidocaine or procainamide); hypokalemia or hypomagnesemia should be corrected.
Ventricular arrhythmias in this setting can rapidly deteriorate into
ventricular tachycardia and fibrillation.
Reversal of Anticoagulation
Once hemostasis is judged acceptable and
the patient continues to remain stable, heparin activ-ity is reversed with
protamine. Protamine is a highly
positively charged protein that binds and effectively inactivates heparin (a
highly negatively charged polysaccharide). Heparin–protamine complexes are then
removed by the reticuloendo-thelial system. Protamine can be dosed in varying
ways, but the results of all techniques should be checked for adequacy by
repeating the ACT 3–5 min after completion of the protamine infusion.
Additional incremental doses of protamine may be necessary.
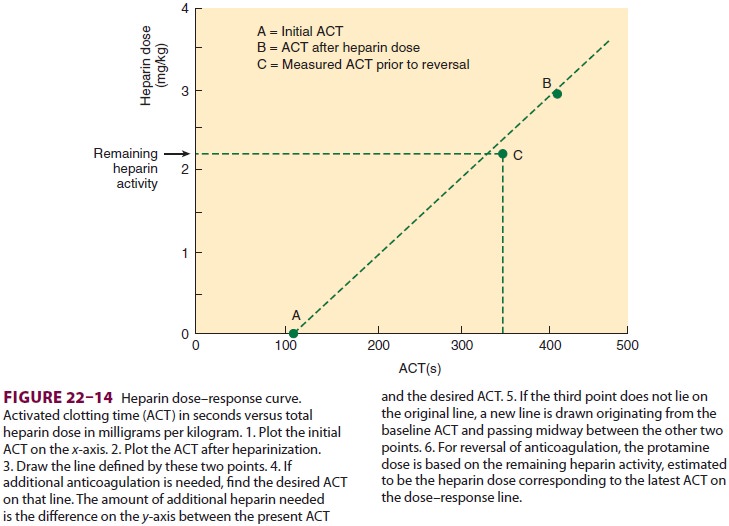
One dosing technique bases the protamine
dose on the amount of heparin initially required to pro-duce the desired ACT;
the protamine is then given in a ratio of 1–1.3 mg of protamine per 100 units
of heparin. A still simpler approach is to give adult patients a defined dose
(eg, 3–4 mg/kg) then check for adequacy of reversal. Another approach
calculates the protamine dose based on the heparin dose–response curve (Figure
22–14). Automated heparin–protamine titration assays effectively mea-sure
residual heparin concentration and can also be used to calculate the protamine
dose. The justifica-tion for using this methodology is the observation that
when protamine is given in excess it may have anticoagulant activity, although
this has never been demonstrated in humans. This approach also assumes that
administered protamine remains in circulation for a prolonged time (which has
been proven false in studies of patients undergoing car-diac surgery). To
accomplish the heparin:protamine titration, premeasured amounts of protamine
are added in varying quantities to several wells, each containing a blood
sample. The well whose prot-amine concentration best matches the heparin
con-centration will clot first. Clotting will be prolonged in wells containing
either too much or too little prot-amine. The protamine dose can then be
estimated by multiplying the concentration in the tube that clots first by the
patient’s calculated blood volume. Supplemental protamine (50–100 mg) should be
considered after administration of unwashed blood remaining in the pump reservoir
after CPB because that blood contains heparin.Protamine administration can
result in a num-ber of adverse hemodynamic effects, some ofwhich are
immunological in origin. Protamine given slowly (5–10 min) usually has few
effects; when given more rapidly it produces a fairly consistent vasodilation
that is easily treated with blood from the pump oxygenator and small doses of
phenyl-ephrine. Catastrophic protamine reactions often include myocardial
depression and marked pul-monary hypertension. Diabetic patients previously
maintained on protamine-containing insulin (such as NPH) may be at increased
risk for adverse reac-tions to protamine.
Persistent Bleeding
Persistent bleeding oft en follows
prolonged durations of bypass (>2 h) and in most instances has multiple
causes. Inadequate surgi-cal control of bleeding sites, incomplete reversal of
heparin, thrombocytopenia, platelet dysfunc-tion, hypothermia-induced
coagulation defects, and undiagnosed preoperative hemostatic defects, or newly
acquired factor deficiency or hypofibri-nogenemia may be responsible. The
absence (or loss) of clot formation may be noted in the surgical field.
Normally, the ACT should return to baseline following administration of
protamine; additional doses of protamine (25–50 mg) may be necessary.
Reheparinization (heparin rebound) after appar-ent adequate reversal is poorly
understood but often attributed to redistribution of peripherally bound heparin
to the central compartment and to the exceedingly short persistence of protamine
in blood. Hypothermia (<35°C) accentuates hemo-static defects and
should be corrected. The admin-istration of platelets and coagulation factors
should be guided by additional coagulation studies, but empiric therapy may be
necessary when such tests are not readily or promptly available as well as when
treating massive transfusion. On the other hand, there can be abnormalities in
multiple tests of coag-ulation whether or not there is bleeding, so the true
diagnostic specificity and reliability of these tests is often overstated.
If diffuse oozing continues despite
adequate surgical hemostasis and the ACT is normal or the heparin–protamine
titration assay shows no residual heparin, thrombocytopenia or platelet
dysfunction is most likely. Comparison of a conventional ACT with an ACT
measured in the presence of hepari-nase (an enzyme that cleaves and inactivates
hepa-rin) can confirm that no residual heparin requiring protamine reversal
remains present when both tests provide the same result. Platelet defects are
recog-nized complications of CPB, which may necessitate platelet transfusion.
Significant depletion of coagu-lation factors, particularly factors V and VIII,
dur-ing CPB is less commonly responsible for bleeding but should be treated
with fresh frozen plasma; both the prothrombin time and partial thromboplas-tin
time are usually prolonged in such instances. Hypofibrinogenemia (fibrinogen
level <100 mg/dL or a prolonged thrombin time without
residual heparin) should be treated with cryoprecipitate. Desmopressin (DDAVP),
0.3 mcg/kg (intravenously over 20 min), can increase the activity of factors
VIII and XII and the von Willebrand factor by releasing them from the vascular
endothelium. DDAVP may be effective in reversing qualitative platelet defects
in some patients but is not recommended for rou-tine use. Accelerated
fibrinolysis may occasion-ally be encountered following CPB and should be
treated with ε-aminocaproic acid or tranexamic acid if one or the
other of these agents has not already being given; the diagnosis should be
confirmed by elevated fibrin degradation products (≥32 mg/mL), or evidence of clot lysis on
thromboelastography. Increasingly, factor VII concentrate (at a cost of many
thousands of dollars) is administered as a “last resort” in the setting of
coagulopathic bleeding fol-lowing cardiac surgery.
Anesthesia
Unless a continuous intravenous infusion
technique is used, additional anesthetic agents are necessary following CPB;
the choice may be determined by the hemodynamic response of the patient
follow-ing CPB. Traditional teaching would have unstable patients receive small
amounts of an opioid, ben-zodiazepine, or scopolamine, whereas anesthetic doses
of a volatile agent might be recommended forhyperdynamic patients.
Nevertheless, we have found that most patients tolerate modest doses of
volatile agents or propofol infusion. Patients with hyper-tension that is
unresponsive to adequate anesthesia with opioids and either a volatile agent or
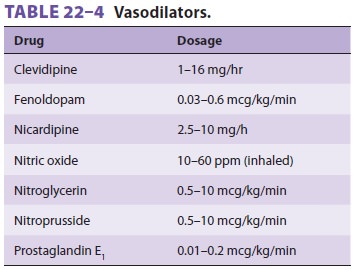
propofol (or both) should receive a vasodilator such as nitro-glycerin,
nitroprusside, clevidipine, or nicardipine (Table 22–4). Fenoldopam may be used
and has the added benefit of increasing renal blood flow which might possibly
improve kidney function in the early postoperative period.
It is common for an opioid (morphine or
hydro-morphone) and either propofol or dexmedetomi-dine to be given to provide
analgesia and sedation during transfer to the ICU and analgesia (after
dis-continuation of the propofol or dexmedetomidine) during emergence.
Transportation
Transporting patients with critical
illness from the operating room to the ICU is a consistently nerve-wracking and
occasionally hazardous process that is complicated by the possibilities of
monitor failure, overdosage or interruption of drug infusions, and hemodynamic
instability en route. Portable moni-toring equipment, infusion pumps, and a
full oxy-gen cylinder with a self-inflating bag for ventilation should be
readied prior to the end of the operation. Minimum monitoring during
transportation includes the ECG, arterial blood pressure, and pulse oximetry. A
spare endotracheal tube, laryngoscope, succinyl-choline, and emergency
resuscitation drugs should also accompany the patient. Upon arrival in the ICU,
the patient should be attached to the ventilator, breath sounds should be
checked, and an orderly transfer of monitors and infusions (one at a time)
should follow. The ICU staff should be given a brief summary of the procedure,
intraoperative problems, current drug therapy, and any expected difficulties.
Many centers insist on a standard protocol for the “hand off.”
Related Topics