Chapter: Clinical Anesthesiology: Anesthetic Management: Anesthesia for Cardiovascular Surgery
Cardiopulmonary Bypass
Cardiopulmonary Bypass
CPB is a technique that diverts venous
blood away from the heart (most often from one or more cannulas in the right
atrium), adds oxygen, removes CO2, and returns the blood through a
cannula in a large artery (usually the ascending aortaor a femoral artery). As
a result, nearly all bloodbypasses the heart and lungs. When CPB is fully
established, the extracorporeal circuit is in series with the systemic
circulation and provides both artificial ventilation and perfusion. This
technique provides distinctly nonphysiological conditions, because arterial
pressure is usually less than normal and blood flow is usually nonpulsatile. To
minimize organ damage during this stressful period, various degrees of systemic
hypothermia may be employed. Topical hypothermia (an ice-slush solution) and
car-dioplegia (a chemical solution for arresting myocar-dial electrical activity)
may also be used to protect the heart.
The operation of the CPB machine is a
complex task requiring the attention of a perfusionist—a spe-cialized (and
certified) technician. Optimal results with CPB require close cooperation and
commu-nication between the surgeon, anesthesiologist, and perfusionist.
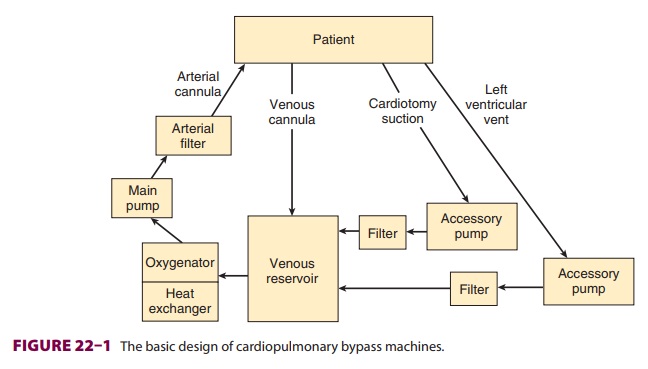
BASIC CIRCUIT
The typical CPB
machine has six basic components: a venous reservoir, an oxygenator, a heat exchanger,a main pump, an arterial filter, tubing
that conducts venous blood to the venous reservoir, and tubingthat conducts
oxygenated blood back to the patient(Figure 22–1). Modern machines use a single
disposable unit that includes the reservoir, oxygen ator, and heat exchanger.
Most machines also have
separate accessory pumps that can be
used for blood salvage (cardiotomy suction), venting (draining) the left
ventricle, and administration of cardioplegia solutions. A number of other
filters, alarms, and in-line pressure, oxygen-saturation, and temperature monitors
are also typically used.
Prior to use, the CPB circuit must be
primed with fluid (typically 1200–1800 mL for adults) that is devoid of
bubbles. A balanced salt solution, such as lactated Ringer’s solution, is
generally used, but other components are frequently added, including colloid
(albumin or starch), mannitol (to promote diuresis), heparin (500–5000 units),
and bicarbon-ate. At the onset of bypass, hemodilution decreases the hematocrit
to about 22–27% in most patients. Blood is included in priming solutions for
smaller children and severely anemic adults to prevent severe hemodilution.
Reservoir
The reservoir of the CPB machine
receives blood from the patient via one or two venous cannulas placed in the
right atrium, the superior and inferior vena cava, or a femoral vein. With most
circuits blood returns to the reservoir by gravity drainage. During
extracorporeal circulation the patient’s venous pressure is normally low. Thus,
the driving force for flow into the pump is directly related to the difference
in height between the patient and the res-ervoir and inversely proportional to
the resistance of the cannulas and tubing. An appropriately primed CPB machine
draws in blood like a siphon. Entrainment of air in the venous line can produce
an air lock that may prevent blood flow. With some cir-cuits (eg, use of an
unusually small venous cannula) assisted venous drainage may be required; a
regu-lated vacuum together with a hard shell venous res-ervoir or centrifugal
pump is used insuch instances. The fluid
level in the reservoir is critical. If a “roller” pump is used and thereservoir
is allowed to empty, air can enter the main pump and be embolized into the
patient where it may cause organ damage or fatality. A low reservoir level
alarm is typically present. Centrifugal pumps will not pump air but have the
disadvantage of not impelling a well-defined volume with each turn of the head
(unlike roller pumps).
Oxygenator
Blood is drained by gravity from the
bottom of the venous reservoir into the oxygenator, which con-tains a blood–gas
interface that allows blood to equilibrate with the gas mixture (primarily
oxygen). A volatile anesthetic is frequently added to the oxy-genator gas
mixture. The blood–gas interface in a modern, membrane-type oxygenator is a very
thin, gas-permeable silicone membrane. Arterial CO 2 tension during CPB is dependent on total gas flow
past the oxygenator. By varying the inspired oxygen concentration, a membrane
oxygenator allows inde-pendent control of Pao2
and Paco2.
Heat Exchanger
Blood from the oxygenator enters the
heat exchanger and can either be cooled or warmed, depending on the temperature
of the water flowing through the exchanger; heat transfer occurs by conduction.
Because gas solubility decreases as blood tempera-ture rises, a filter is built
into the unit to catch any bubbles that may form during rewarming.
Main Pump
Modern CPB machines use either an
electrically driven double-arm roller (positive displacement) or a centrifugal
pump to propel blood through the CPB circuit.
A. Roller Pumps
Roller pumps produce flow by compressing
large-bore tubing in the main pumping chamber as the roller heads turn.
Subtotal occlusion of the tubing prevents excessive red cell trauma. The
rollers pump blood regardless of the resistance encountered, and produce a
nearly continuous nonpulsatile flow. Flow is directly proportional to the
number of revolutions per minute. In some pumps, an emergency back-up battery
provides power in case of an electrical power failure. All roller pumps have a
hand crank to allow manual pumping, but those who have hand cranked a roller
pump head will confirm that this is not a good long-term solution.
B. Centrifugal Pumps
Centrifugal pumps consist of a series of
cones in a plastic housing. As the cones spin, the centrifugal forces created
propel the blood from the centrally located inlet to the periphery. In contrast
to roller pumps, blood flow with centrifugal pumps is pres-sure sensitive and
must be monitored by an elec-tromagnetic flowmeter. Increases in distal
pressure will decrease flow and must be compensated for by increasing the pump
speed. Because these pumps are nonocclusive, they are less traumatic to blood
than roller pumps. Unlike roller pumps, which are placed after the oxygenator
(Figure 22–1), centrifugal pumps are normally located between the venous
reservoir and the oxygenator. Centrifugal (unlike roller) pumps have the
advantage of not being able to pump air.
C. Pulsatile Flow
Pulsatile blood flow is possible with
some roller pumps. Pulsations can be produced by instantaneous variations in
the rate of rotation of the roller heads; they can also be added after flow is
generated. Pulsatile flow is not available with centrifugal pumps. Although
there is no consensus and the data are contradictory, some clinicians believe
that pulsatile flow improves tissue perfusion, enhances oxygen extraction,
attenu-ates the release of stress hormones, and results in lower systemic
vascular resistances (SVRs) during CPB.
Arterial Filter
Particulate matter (eg, thrombi, fat
globules, tissue debris) may enter the CPB circuit via the cardiotomy suction
line. Although filters are often used at other locations, a final, in-line,
arterial filter (27–40 μm)
helps to reduce systemic embolism. Once filtered, the propelled blood returns
to the patient, usually via a cannula in the ascending aorta, or less com-monly
in the femoral artery. A normally functioning aortic valve prevents blood from
regurgitating into the left ventricle.
The filter is always in parallel with a
(normally clamped) bypass limb in case the filter becomes clogged or develops
increased resistance. For the same reason, arterial inflow pressure is measured
before the filter. The filter is also designed to trap air, which can be bled
out through a built-in stopcock.
Accessory Pumps & Devices
A. Cardiotomy Suction
The cardiotomy suction pump aspirates
blood from the surgical field during CPB and returns it directly to the main
pump reservoir. This is a potential port of entry for fat and other debris to
the pump that could embolize to organs. A so-called cell-saver suc-tion device
may also be used to aspirate blood from the surgical field, in which case blood
is returned to a separate reservoir on a separate device. When sufficient blood
has accumulated (or at the end of the procedure), the cell-saver blood is
centrifuged, washed, and returned to the patient. Excessive suc-tion pressure
can theoretically contribute to red cell trauma. Use of cell-saver suction
(instead of cardi-otomy suction) during bypass will deplete CPB cir-cuit volume
if blood loss is brisk. The high negative pressure of ordinary wall suction
devices produces excessive red cell trauma precluding blood salvage from that
source.
B. Left Ventricular Vent
With time, even with “total” CPB, blood
reaccumu-lates in the left ventricle as a result of residual pulmo-nary flow
from the bronchial arteries (which arise directly from the aorta or the
intercostal arteries) or thebesian vessels , or sometimes as a result of aortic
valvular regurgitation. Aortic regurgitation can occur as a result of either
(struc-tural) valvular abnormalities or surgical manipula-tion of the heart
(functional). Distention by blood of the left ventricle compromises myocardial
pres-ervation and requires decompression
(venting). Most surgeons accomplish this by insert-ing a catheter via the right
superior pulmonary vein and left atrium into the left ventricle. Venting may
also be accomplished using a catheter placed in the left ventricular apex or
across the aortic valve. The blood aspirated by the vent pump normally passes
through a filter before being returned to the venous reservoir.
C. Cardioplegia Pump
Cardioplegic solutions are most often
administered via an accessory pump on the CPB machine. This technique allows
optimal control over the infusion pressure, rate, and temperature. A separate
heat exchanger ensures control of the temperature of the cardioplegia solution.
Less commonly, cardioplegic solutions may be infused from a cold intravenous
fluid bag given under pressure or by gravity.
Ultrafiltration
Ultrafiltration can be used during CPB
to increase the patient’s hematocrit without transfusion. Ultrafilters consist
of hollow capillary fibers that can function as membranes, allowing separation
of the aqueous phase of blood from its cellular and pro-teinaceous elements.
Blood can be diverted to pass through the fibers either from the arterial side
of the main pump or from the venous reservoir using an accessory pump.
Hydrostatic pressure forces water and electrolytes across the fiber membrane.
Effluents of up to 40 mL/min may be removed.
Related Topics