Chapter: Clinical Anesthesiology: Anesthetic Management: Anesthesia for Cardiovascular Surgery
Anesthetic Management of Cardiac Surgery: Preinduction Period
Preinduction Period
Premedication
The prospect of heart surgery is
frightening, and relatively “heavy” oral or intramuscular premedica-tion was
often given in the past, particularly when patients had coronary artery disease
with good left ventricular function . However, in current practice, most
patients receive no sedative-hypnotic premedication until their arrival on the
surgical unit, at which time most will receive small doses of intravenous
midazolam.
Benzodiazepine sedative-hypnotics
(diazepam, 5–10 mg orally), alone or in combination with an opioid (morphine,
5–10 mg intramuscularly or hydromorphone, 1–2 mg intramuscularly), were often
used in the past. Longer acting premedicant agents (eg, lorazepam) are avoided
by most practi-tioners to permit “fast tracking” of patients through their
recovery.
Preparation
The best practitioners of cardiac
anesthesia formu-late a simple anesthetic plan that includes adequate
preparations for contingencies. Many patients are critically ill, and there is
little time intraoperatively to have an assistant search for drugs and
equipment. At the same time, the anesthetic plan should not be excessively rigid;
when problems are encountered with one technique, one should be ready to change
to another without delay. Preparation, organization, and attention to detail
permit one to more efficiently deal with unexpected intraoperative problems.
The anesthesia machine, monitors, infusion pumps, and blood warmer should all
be checked before the patient arrives. Drugs—including anesthetic and
vasoactive agents—should be immediately avail-able. Many clinicians prepare one
vasoconstrictor and one vasodilator infusion before the start of the procedure.
Venous Access
Cardiac surgery is sometimes associated
with large and rapid blood loss, and with the need for multiple drug infusions.
Ideally, two large-bore (16-gauge or larger) intravenous catheters should be
placed. One of these should be in a large central vein, usually an internal or
external jugular or subclavian vein. Central venous cannulations may be
accomplished while the patient is awake but sedated or after induc-tion of
anesthesia. Studies show no benefit from placing either central venous or
pulmonary arterial catheters in awake (versus anesthetized) patients undergoing
cardiovascular surgery.
Drug infusions should ideally be given
into a central catheter, preferably directly into the catheter or into the injection
port closest to the catheter (to minimize dead space). Multilumen central
venous catheters and multilumen pulmonary artery cathe-ter introducer sheaths
allow for multiple drug infu-sions with simultaneous measurement of vascular
pressures. One intravenous port should be dedicated for drug infusions and
nothing else; drug and fluid boluses should be administered through another
site. The side port of the introducer sheath used for a pulmonary catheter can
be used for drug infusions but serves better as a fluid bolus line when a
large-bore introducer (9F) is used.Blood should be immediately available for
transfusion if the patient has already had amidline sternotomy (a “redo”); in
these cases, the right ventricle or coronary grafts may be adherent to the
sternum and may be accidentally entered during the repeat sternotomy.
Monitoring
A. Electrocardiography
The electrocardiogram (ECG) is
continuously monitored with two leads, usually leads II and V5. Baseline tracings of all leads may be recorded on
paper for further reference. The advent of monitors with computerized
ST-segment analysis and the use of additional monitoring leads (V 4, aVF, and V4R)
have greatly improved detection of ischemic epi-sodes, as has the frequent
intraoperative use of TEE.
B. Arterial Blood Pressure
In addition to all basic monitoring,
arterial cannula-tion is always performed either prior to or immedi-ately after
induction of anesthesia, as the induction period represents a time when major
hemodynamic alterations may occur. Radial arterial catheters may occasionally
give falsely low readings following ster-nal retraction as a result of
compression of the sub-clavian artery between the clavicle and the first rib.
They may also provide falsely low values early after CPB due to the opening of
atrioventricular shunts in the hand during rewarming. The radial artery on the
side of a previous brachial artery cutdown should be avoided, because its use
is associated with a greater incidence of arterial thrombosis and wave
distor-tion. Obviously, if a radial artery will be harvested for a coronary
bypass conduit, it cannot be used as a site for arterial pressure monitoring.
Other useful catheterization sites include the ulnar, axillary, and especially
brachial and femoral arteries. A backup manual or automatic blood pressure cuff
should also be placed on the opposite side for comparison with direct
measurements.
C. Central Venous and Pulmonary Artery Pressure
Central venous pressure is not terribly
useful for diagnosis of hypovolemia but has been customarily monitored in
nearly all patients undergoing cardiac surgery. The decision about whether to
use a pulmo-nary artery catheter is based on the patient, the pro-cedure, and
the preferences of the surgical team. Routine use of a pulmonary artery
catheter, once nearly universal in adult cardiovascular practice, is
controversial. Pulmonary artery catheterization has declined precipitously in
nearly all circumstances except adult cardiac surgery due to lack of evidence
of a positive effect on patient outcomes. Left ven-tricular filling pressures
can be measured with a left atrial pressure line inserted by the surgeon
duringbypass. In general, pulmonary artery catheter-ization has been most of
ten used in patientswith compromised ventricular function (ejection fraction <40–50%) or pulmonary hypertension and in those
undergoing complicated procedures. The most useful data are pulmonary artery
pressures, the pulmonary artery occlusion (“wedge”) pressure, and
thermodilution cardiac outputs. Specialized cathe-ters provide extra infusion
ports, continuous mea-surements of mixed venous oxygen saturation and cardiac
output, and the capability for right ventricu-lar or atrioventricular
sequential pacing. Given the risk associated with placing any pulmonary artery
catheter, some clinicians opine that it makes sense to restrict pulmonary
artery catheterization only to devices that offer these advanced capabilities.
The right internal jugular vein is the
preferred approach for intraoperative central venous can-nulation. Catheters
placed through the other sites, particularly on the left side, are more likely
to kink following sternal retraction (above) and are not nearly as likely to
pass into the superior vena cava as those placed through the right internal
jugular vein.
Pulmonary artery catheters migrate
distally during CPB and may spontaneously wedge with-out balloon inflation.
Inflation of the balloon under these conditions can rupture a pulmonary artery
causing lethal hemorrhage. Pulmonary artery cath-eters should be routinely retracted
2–3 cm during CPB and the balloon subsequently inflated slowly. If the catheter
wedges with less than 1.5 mL of air in the balloon, it should be withdrawn
farther.
D. Urinary Output
Once the patient is anesthetized, an
indwelling uri-nary catheter is placed to monitor the hourly output. Bladder
temperature is often monitored as a mea-sure of core temperature but may not
track core tem-perature well with reduced urinary flow. The sudden appearance
of reddish urine may indicate excessive red cell hemolysis caused by CPB or a
transfusion reaction.
E. Temperature
Multiple temperature monitors are
usually placed once the patient is anesthetized. Bladder (or rectal),
esophageal, and pulmonary artery (blood) tempera-tures are often simultaneously
monitored. Because of the heterogeneity of readings during cooling and
rewarming, bladder and rectal readings are gener-ally taken to represent an
average body temperature, whereas esophageal represents core temperature.
Pulmonary artery temperature provides an accurate estimate of blood
temperature, which should be the same as core temperature in the absence of
active cooling or warming. Nasopharyngeal and tympanic probes may most closely
approximate brain tem-perature. Myocardial temperature is often measured directly
during CPB.
F. Laboratory Parameters
Intraoperative laboratory monitoring is
mandatory during cardiac surgery. Blood gases, hematocrit, serum potassium,
ionized calcium, and glucose measurements should be immediately available. The activated clotting time (ACT)
approximates the Lee–White clotting time and is used to moni-tor heparin
anticoagulation. Some centers routinely use thromboelastography (TEG) to
identify causes of bleeding after CPB.
G. Surgical Field
One of the most important actions in
intraopera-tive monitoring is inspection of the surgical field. Once the
sternum is opened, lung expansion can be observed through the pleura. When the
pericardium is opened, the heart (primarily the right ventricle) is visible;
thus cardiac rhythm, volume, and contractil-ity can often be judged visually.
Blood loss and sur-gical maneuvers must be closely watched and related to
changes in hemodynamics and rhythm.
H. Transesophageal Echocardiography
TEE provides valuable information about
car-diac anatomy and function during surgery.Two-dimensional, multiplane TEE
can detect regional and global ventricular abnormalities, cham-ber dimensions,
valvular anatomy, and the presence of intracardiac air. Three-dimensional TEE
provides a more complete description of valvular anatomy and pathology. TEE can
also be helpful in confirm-ing cannulation of the coronary sinus for
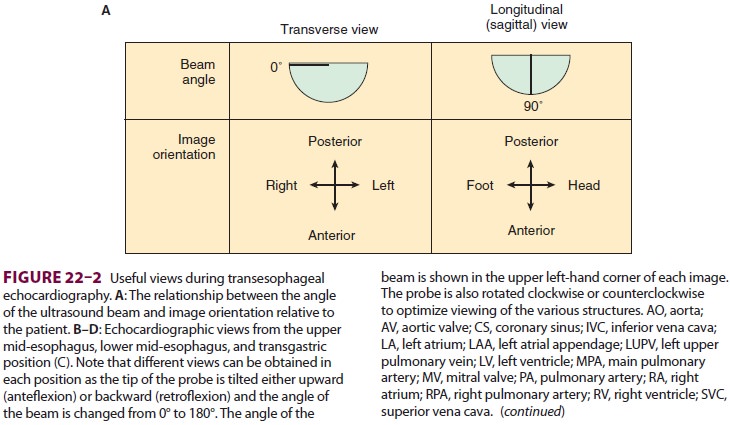
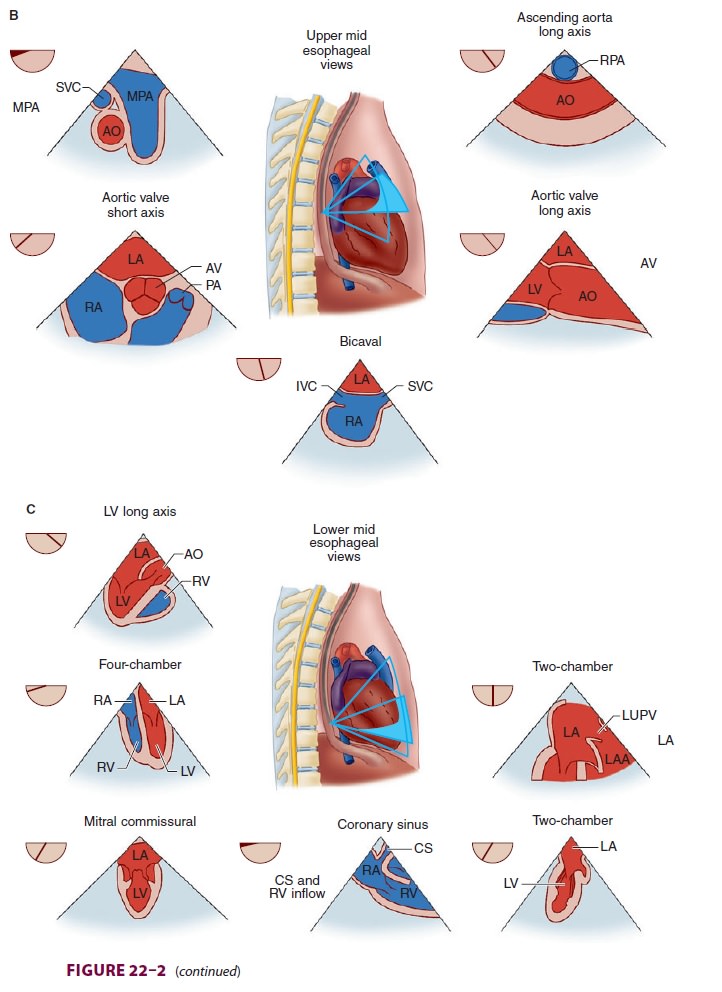
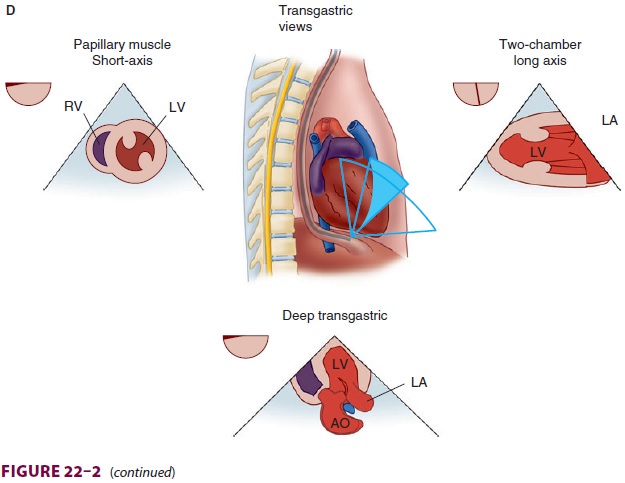
cardiople-gia. Multiple views should be obtained from the upper esophagus,
mid-esophagus, and transgastric positions in the transverse, sagittal, and in-between
planes (Figure
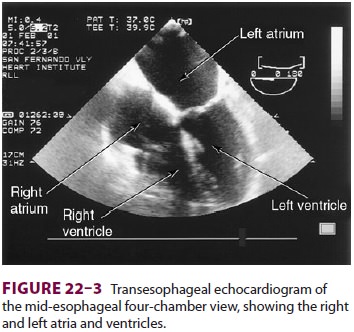
22–2). The two views most com-monly used for monitoring during
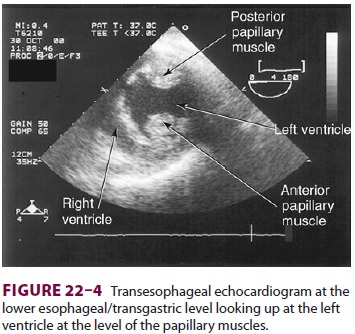
cardiac surgery are the four-chamber view (Figure 22–3) and the transgastric (short-axis)
view (Figure
22–4). Three-dimensional echocardiography offers great promise for
better visualization of complex anatomic fea-tures, particularly of cardiac
valves. The following represent the most important applications of
intra-operative TEE.
1.
Assessment of valvular function—Valvular
mor-phology can be assessed by multiplane and three-dimensional TEE. Pressure
gradients, area and sever-ity of stenosis, and severity of valvular
regurgitation can be assessed by Doppler echocardiography and
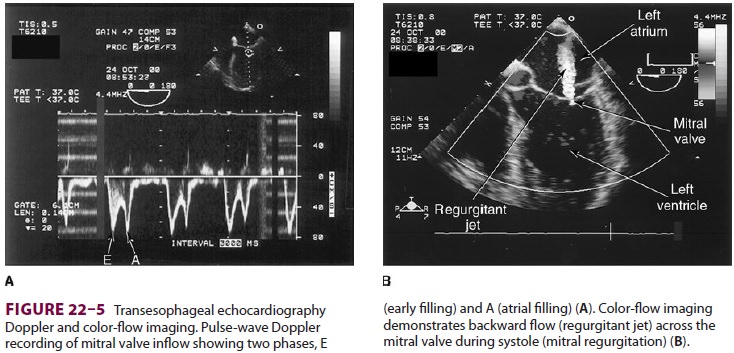
color-fl ow imaging ( Figure 22–5 ). Colors are usually adjusted so that fl ow toward the probe is red and fl
ow in the opposite direction is blue. TEE also can detect prosthetic valve
dysfunction, such as obstruction or regurgitation, and can detect vegetations
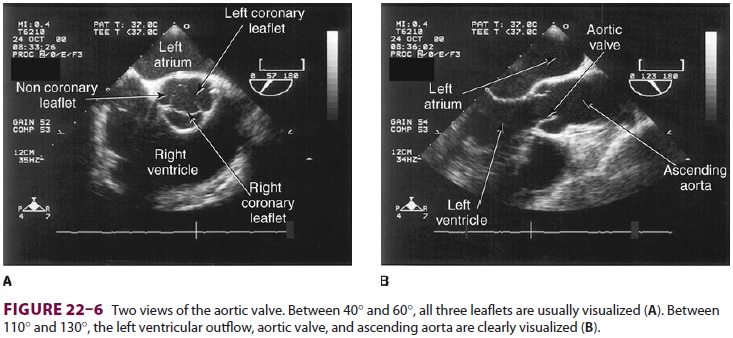
from endocarditis. The TEE images in the upper mid-esophagus at 40–60° and 110–130°
are useful for examining the aortic valve and
ascending aorta ( Figure 22–6 ). The
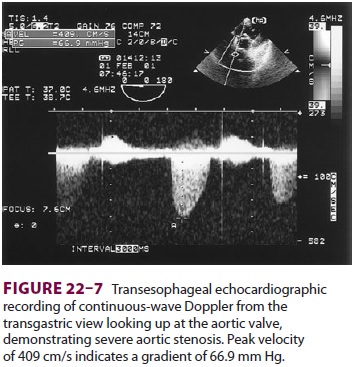
valve annular diameter can also be estimated
with reasonable accuracy. Doppler flow across the aortic valve must be measured
looking up from the deep transgastric view ( Figure 22–7). The anatomic
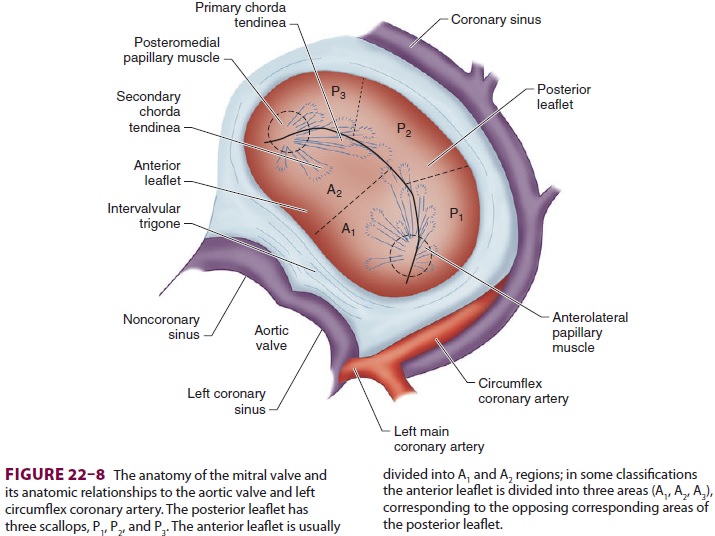
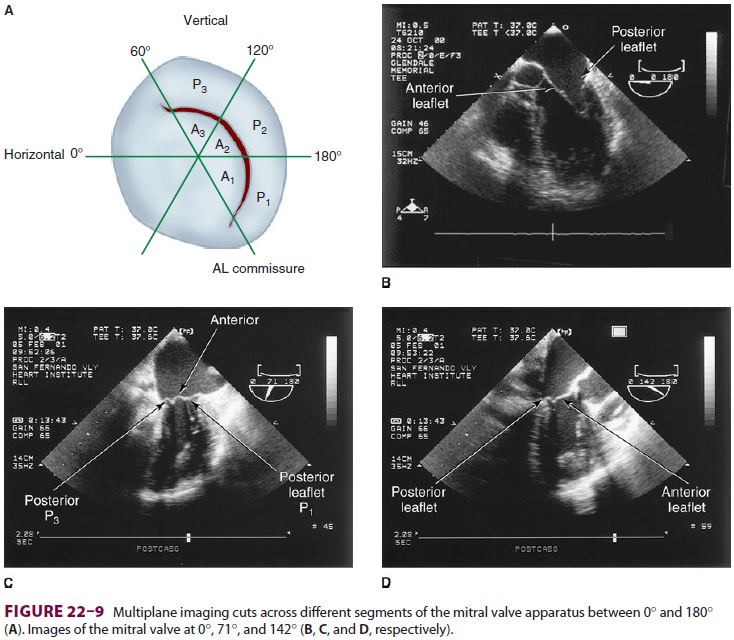
fea-tures of the mitral valve relevant to TEE are shown in Figure 22–8. The mitral valve is
examined from themid-esophageal position, looking at the mitral valve apparatus
with and without color in the 0° through 150° views (Figure 22–9). TEE is an invaluable aid to
guide and assess the quality of mitral valve repair surgery. The commissural
view (at about 60°) is par-ticularly helpful because it
cuts across many scallops of the mitral valve.
2.
Assessment of ventricular function—Ventricularfunction can be assessed by global
systolic function, estimated by means of ejection fraction (often cal-culated
using Simpson’s method of disks) and left ventricular end-diastolic volume;
diastolic function
(ie, looking for abnormal relaxation and
restrictive diastolic patterns by checking mitral flow velocity or by measuring
movements of the mitral valve annulus using tissue Doppler techniques); and
regional systolic function (by assessing wall motion and thickening
ab-normalities). Regional wall abnormalities from myo-cardial ischemia often
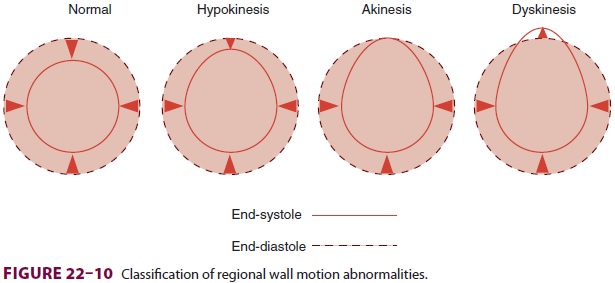
appear before ECG changes. Regional wall motion abnormalities can be classified
into three categories based on severity (Figure 22–10): hypokinesis (reduced wall
motion), akinesis (no wall motion), and dyskinesis (paradoxical wall motion).
The location of a regional wall motion abnormal-ity can indicate which coronary
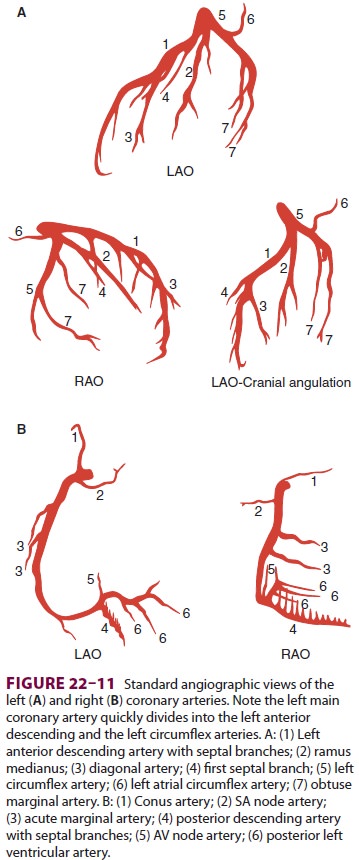
artery is experienc-ing reduced flow. The left ventricular myocardium is
supplied by three major arteries: the left anterior descending artery, the left
circumflex artery, and the right coronary artery (Figure 22–11). The areas of
dis-tribution of these arteries on echocardiographic views
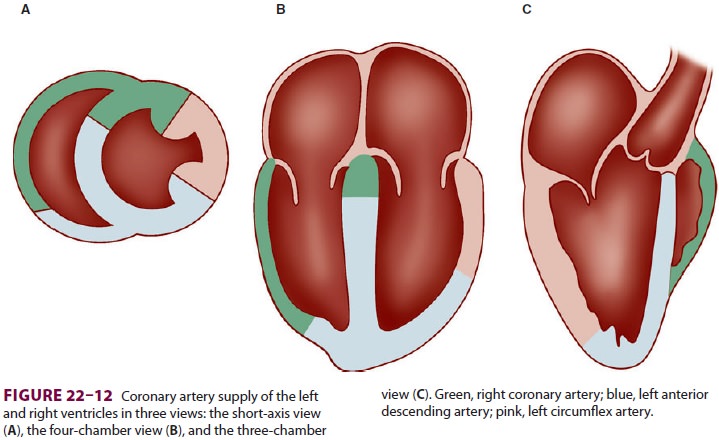
are shown in Figure 22–12. The ventricular
short-axis mid view at the mid-papillary muscle level con-tains all three blood
supplies from the major coronary arteries.
3. Assessment of other cardiac
structures and abnormalities—In an adult undergoing elective cardiac surgery
TEE can help diagnose previously undetected congenital defects such as an
atrial or ventricular septal defect; pericardial diseases such as pericardial
effusions and constrictive pericarditis; and cardiac
tumors. Doppler color-flow imaging helps delineate abnormal intracardiac blood
flows and shunts. TEE can assess the extent of myomectomy in patients with
hypertrophic car diomyopathy (idiopathic hypertrophic subaortic
stenosis). Upper-, mid-, and lower-esophageal views are valuable in diagnosing
aortic disease processes such as dissection, aneurysm, and atheroma (Figure 22–13). The extent of dissections in the ascending and descending
aorta can be accurately defined; however, airway structures prevent complete
visualization of the aortic arch. The presenceof protruding atheroma in the
ascending aorta increases the risk of postoperative stroke and should prompt
the use of epiaortic scanning to identify an atheroma-free cannulation site or
a change in surgical plans.
4.
Examination for residual air—Air
is introduced into the cardiac chambers during all “open” heart procedures,
such as valve surgery. Residual amounts of air often remain in the left
ventricular apex even after the best deairing maneuvers. TEE is helpful in
defining the volume of residual air, to determine whether additional surgical
maneuvers need to be undertaken to help avoid cerebral or coronary embolism.
I. Electroencephalography
Computer-processed electroencephalographic (EEG) recordings can be used to assess anesthetic depth during cardiac surgery, and either the processed or “raw” EEG can be used to ensure complete drug-induced electrical silence (for brain protection) prior to circulatory arrest. These recordings are gen-erally not useful in detecting neurological insults during CPB. Progressive hypothermia (or progres-sively deepened anesthesia) is typically associated with EEG slowing, burst suppression, and, finally, an isoelectric recording. Most strokes during CPB are due to small emboli that are not likely to pro-duce changes in the EEG. Artifacts from the CPB roller pump may be seen on the raw EEG (due to piezoelectric effects from compression of the pump tubing) but can usually be identified as such by com-puter processing.
J. Transcranial Doppler (TCD)
This modality provides noninvasive
measurements of blood flow velocity in the middle cerebral artery, which is
insonated through the temporal bone. TCD is useful for detecting cerebral
emboli. Increased numbers of emboli detected by TCD or Doppler interrogation of
the carotid artery have been asso-ciated with an increased risk of
postoperative neu-robehavioral dysfunction.
Induction of Anesthesia
Cardiac operations usually require
general anesthe-sia, endotracheal intubation, and controlled ven-tilation. Some
centers have used thoracic epidural anesthesia alone for minimally invasive
surgery without CPB or combined thoracic epidural with light general
endotracheal anesthesia for other forms of cardiac surgery. These techniques
have never been popular in North America due to concerns about the risk of
spinal hematomas following heparinization, the associated medical–legal
consequences, and the limited evidence of an outcome benefit. Other cen-ters
use a single intrathecal morphine injection to provide postoperative analgesia.
For elective procedures, induction of
general anesthesia should be performed in a smooth, con-trolled (but not
necessarily “slow”) fashion often referred to as a cardiac induction. Selection
of anesthetic agents is generally less important than the way they are used.
Indeed, studies have failed to show differ-ences in long-term outcome with
various anesthetictechniques. Anesthetic dose requirements are variable and
patient tolerance of inhaled anesthetics generally declines with declining
ventricular function. Severely compromised patients should be given anesthetic
agents in incremental, small doses. A series of challenges may be used to judge
when anesthetic depth will allow intubation without a marked hypertensive
response, while also avoiding hypotension from excessive anesthetic dosing.
Blood pressure and heart rate are continuously evaluated following
unconsciousness, insertion of an oral air-way, urinary catheterization, and
tracheal intuba-tion. A sudden increase in heart rate or blood pressure may
indicate light anesthesia and the need for more anesthetic prior to the next
challenge, whereas a decrease or no change suggests that the patient is ready
for the subsequent stimulus. Muscle relaxant is given after consciousness is
lost. Reductions in blood pressure greater than 20% gen-erally call for administration
of a vasopressor .
The period following intubation is often
char-acterized by a gradual decrease in blood pressure resulting from the
anesthetized state (often associ-ated with vasodilation and decreased
sympathetic tone) and a lack of surgical stimulation. Patients will usually
respond to fluid boluses or a vasocon-strictor. Nevertheless, the
administration of large amounts of intravenous fluids prior to the bypass may
serve to accentuate the hemodilution asso-ciated with CPB (below). Small doses
of phenyl-ephrine (25–100 mcg), vasopressin (1–3 units), or ephedrine (5–10 mg)
may be useful to avoid excessive hypotension. Following intubation and
institution of controlled ventilation; arterial blood gases, hematocrit, serum
potassium, and glu-cose concentrations are measured. The baseline ACT (normal <130 s) is best measured after skin incision.
Choice of Anesthetic Agents
Anesthetic techniques for cardiac
surgery have evolved over the years. Successful techniques range from primarily
volatile inhalation anesthesia to high-dose opioid totally intravenous
techniques. In recent years, total intravenous anesthesia with short-acting
agents and combinations of intra-venous and volatile agents have become most
popular.
A. “High-Dose” Opioid Anesthesia
Th is technique was originally developed
to circum-vent the myocardial depression associated with older volatile
anesthetics, particularly halothane. But pure high-dose opioid anesthesia (eg,
fentanyl, 50–100 mcg/kg, or sufentanil, 15–25 mcg/kg) pro-duces prolonged
postoperative respiratory depres-sion (12–24 h), is associated with an
unacceptably high incidence of patient awareness (recall) during surgery, and
often fails to control the hypertensive response to stimulation in many
patients with pre-served left ventricular function. Other undesirable effects
include skeletal muscle rigidity during induc-tion and prolonged postoperative
ileus. Moreover, simultaneous administration of benzodiazepines with large
doses of opioids can produce hypotension and myocardial depression. Patients
anesthetized with sufentanil (and other shorter acting agents) generally regain
consciousness and can be extubated sooner than those anesthetized with
fentanyl.
B. Total Intravenous Anesthesia (TIVA)
The drive for cost containment in
cardiac surgery was a major impetus for development of anesthe-sia techniques
with short-acting agents. Although the drugs may be costlier, large economic
benefits resulted from earlier extubation, decreased inten-sive care unit (ICU)
stays, earlier ambulation, and earlier hospital discharge (“fast-track”
manage-ment). One technique employs induction with pro-pofol (0.5–1.5 mg/kg
followed by 25–100 mcg/kg/ min), and modest doses of fentanyl (total doses of
5–7 mcg/kg) or remifentanil (0–1 mcg/kg bolus fol-lowed by 0.25–1 mcg/kg/min).
Target controlled infusion (TCI) employs software and hardware (computerized
infusion pump) to deliver a drug and achieve a set concentration at the effect
site basedon pharmacokinetic modeling. For propofol the clinician sets only the
patient’s age and weight, and the desired blood concentration on the
Diprifusor, a TCI device widely available in countries outside North America.
During cardiac surgery, this tech-nique can be used for propofol with a target
con-centration of 1.5–2 mcg/mL. Whenever the very short-acting remifentanil is
used for painful surgery, provision must be made for postoperative analgesia
after its discontinuation.
C. Mixed Intravenous/Inhalation Anesthesia
Renewed interest in volatile agents came
about fol-lowing studies demonstrating the protective effects of volatile
agents on ischemic myocardium and an increased emphasis on fast-track recovery
of car-diac patients. Selection of anesthetic agents is ori-ented to
hemodynamic stability as well as early extubation (1–6 h). Propofol (0.5–1.5
mg/kg) or etomidate (0.1–0.3 mg/kg) is often used for induc-tion. Induction
usually follows sedation with small doses of midazolam (0.05 mg/kg). Opioids
are given in small doses together with a volatile agent (0.5–1.5 minimum
alveolar concentration [MAC]) for maintenance anesthesia and to blunt the
sym-pathetic response to stimulation. The opioid may be given in small
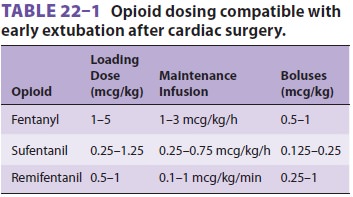
intermittent boluses, by continuous infusion, or both (Table 22–1). To facilitate
fast-track management, typical total doses of fentanyl and sufentanil generally
do not exceed 15 and 5 mcg/kg, respectively, and some clinicians com-bine much
smaller doses of fentanyl or sufentanil with an analgesic dose of hydromorphone
or mor-phine administered toward the end of CPB. Some clinicians also
administer a low-dose infusion of propofol (25–50 mcg/kg/min) for maintenance.
The major advantage of volatile agents
or infusions of remifentanil or propofol, or both, is the ability to change the
anesthetic concentration and depth rapidly. Isoflurane, sevoflurane, and
desflurane are the most commonly used volatile anesthetics. Early laboratory
reports of isoflurane inducing intracoro-nary steal have been overshadowed by
later reports of myocardial protection. Isoflurane remains a com-monly used
volatile agent. Nitrous oxide is gener-ally not used, particularly during the
time interval between cannulation and decannulation, because of its tendency to
expand any intravascular air bubbles that may form.
D. Other Techniques
The combination of ketamine with
midazolam (or diazepam or propofol) for induction and mainte-nance of
anesthesia is a useful technique, particularly in frail patients with
hemodynamic compromise. It is associated with stable hemodynamics, reli-able
amnesia and analgesia, minimal postoperative respiratory depression, and rare
(if any) psychoto-mimetic side effects. Ketamine and midazolam are compatible
in solution, and may be mixed together in the same syringe or infusion bag in a
20:1 ratio. For induction, ketamine, 1–2 mg/kg, with mid-azolam, 0.05–0.1
mg/kg, is given as a slow intrave-nous bolus. Anesthesia can then be maintained
by infusion of ketamine, 1.3–1.5 mg/kg/h, and mid-azolam, 0.065–0.075 mg/kg/h,
or more easily with an inhaled agent. Hypertension following intubation or
surgical stimulation can be treated with propofol or a volatile agent.
E. Muscle Relaxants
Muscle relaxation is helpful for
intubation, to facilitate sternal retraction, and to prevent patient movement
and shivering. Unless airway difficul-ties are expected, intubation may be
accomplished after administration of a nondepolarizing mus-cle relaxant. The
choice of muscle relaxant in the past was often based on the desired
hemodynamic response. Modern, shorter acting agents such as rocuronium,
vecuronium, and cisatracurium are commonly used and have almost no hemodynamic
side effects of their own. Vecuronium, however, has been reported to markedly
enhance bradycardia associated with large doses of opioids, particularly
sufentanil. Because of its vagolytic effects, pan-curonium was often used in
patients with marked bradycardia who were taking β-blocking agents, Succinylcholine
remains appropriate for endotra-cheal intubation, particularly for rapid
sequence induction. Judicious dosing and appropriate use ofperipheral nerve
stimulator allow fast-tracking with any of these agents.
Related Topics