Chapter: Clinical Anesthesiology: Anesthetic Management: Anesthesia for Cardiovascular Surgery
Anesthetic Management of Cardiac Surgery: Termination of CPB
Termination of CPB
Discontinuation of bypass is
accomplished by a series of necessary procedures and conditions:
·
Rewarming
must be completed.
·
Air
must be evacuated from the heart and any bypass grafts.
·
The
aortic cross-clamp must be removed and the heart must beat.
·
Lung
ventilation must be resumed.
The surgeon’s decision about when to
rewarm is important; adequate rewarming requires time, but rewarming too soon
removes the protective effects of hypothermia. Rapid rewarming often results in
large temperature gradients between well-perfused organs and peripheral
vasoconstricted tissues; sub-sequent equilibration following separation from
CPB decreases core temperature again. An excessive gradient between the
infusate temperature and the patient’s core temperature can result in
deleterious brain hyperthermia. Infusion of a vasodilator drug (nitroprusside,
isoflurane, or phentolamine [primar-ily in children]) by allowing higher pump
flows often speeds the rewarming process and decreases large temperature
gradients. Some believe that allowing some pulsatile flow (ventricular
ejection) may also speed rewarming. Excessively rapid rewarming, however, can
result in the formation of gas bubbles in the bloodstream as the solubility of
gases rapidly decreases. If the heart fibrillates during rewarm-ing, direct
electrical defibrillation (5–10 J) may be necessary. Administration of
lidocaine, 100–200 mg, and magnesium sulfate, 1–2 g, prior to removal of aortic
cross-clamping is a common protocol and may decrease the likelihood of fibrillation.
Many clinicians advocate a head-down position while intracardiac air is being
evacuated to decrease the likelihood of cerebral emboli. Lung inflation
facili-tates expulsion of (left-sided) intracardiac air by compressing
pulmonary vessels and returning blood into the left heart. TEE is useful in
detecting resid-ual intracardiac air. Initial reinflation of the lungs requires
greater than normal airway pressure and should generally be done under direct
visualization of the surgical field because excessive lung expansion can
interfere with internal mammary artery grafts.
General guidelines for separation from
CPB include the following:
·
The
core body temperature should be at least 37°C.
·
A
stable rhythm must be present. Atrioventricular pacing is often used and
confers the benefit of a properly timed atrial systole. Persistence of
atrioventricular block should prompt measurement of serum potassium
concentration. If hyperkalemia is present, it can be treated with calcium,
NaHCO3, furosemide, or glucose and insulin.
·
The
heart rate must be adequate (generally 80–100 beats/min). Slow heart rates are
generally treated by pacing. Many inotropic agentswill also increase heart
rate. Supraventricular tachycardias generally require cardioversion.
·
Laboratory
values must be within acceptable limits. Significant acidosis (pH < 7.20), hypocalcemia (ionized), and hyperkalemia (>5.5 mEq/L) should be treated; ideally the hematocrit
should exceed 22%; however, a hematocrit <22% should not by itself trigger
transfusion of red blood cells at this time. When CPB reservoir volume and flow
are adequate, ultrafiltration may be used to increase the hematocrit.
·
Adequate
ventilation with 100% oxygen must have been resumed.All monitors should be
rechecked for proper function and recalibrated if necessary.
Weaning from CPB
CPB should be discontinued as systemic
arterial pressure, ventricular volumes and filling pressures, and cardiac
function (on TEE) are assessed. Central aortic pressure may be measured
directly and should be compared with the radial artery pressure and cuff
pressure (if there is a disparity). A reversal of the normal systolic pressure
gradient, with aortic pressure being greater than radial pressure, is often
seen immediately postbypass. This has been attrib-uted to opening of
arteriovenous connections in the hand as a consequence of rewarming. Central
aortic root pressure can also be estimated by palpation by an experienced
surgeon. Right ventricular volume and contractility can be estimated visually,
whereas filling pressures are measured directly by central venous, pulmonary
artery, or left atrial catheters. Cardiac output can be measured by
thermodilution. TEE can define adequacy of end-diastolic volumes, right and
left ventricular contractility, and valvular function.
Weaning is typically accomplished by
pro-gressively clamping the venous return line (tub-ing). As the beating heart
fills, ventricular ejection resumes. Pump flow is gradually decreased as
arterial pressure rises. Once the venous line is completely occluded and
systolic arterial pressure is judged to be adequate (>80–90 mm Hg), pump flow is stopped and the patient
is evaluated. Some surgeons wean by clamping the venous line and then
progressively “filling” the patient with arte-rial inflow.
Most patients fall into one of four
groups when coming off bypass ( Table 22–2).
Patients with good ventricular function are usually quick to develop good blood
pressure and cardiac out-put and can be separated from CPB immedi-ately.
Hyperdynamic patients can also be rapidly weaned. These patients emerge from
CPB with a very low SVR, demonstrating good contractility and adequate volume,
but have low arterial pres-sure; their hematocrit is often reduced (<22%). Diuresis (off CPB) or red blood cell
transfusions increase arterial blood pressure.
Hypovolemic patients include those with
nor-mal ventricular function and those with varying degrees of impairment.
Those with preserved myo-cardial function quickly respond to 100-mL aliquots of
pump blood infused via the aortic cannula. Blood pressure and cardiac output
rise with each bolus,
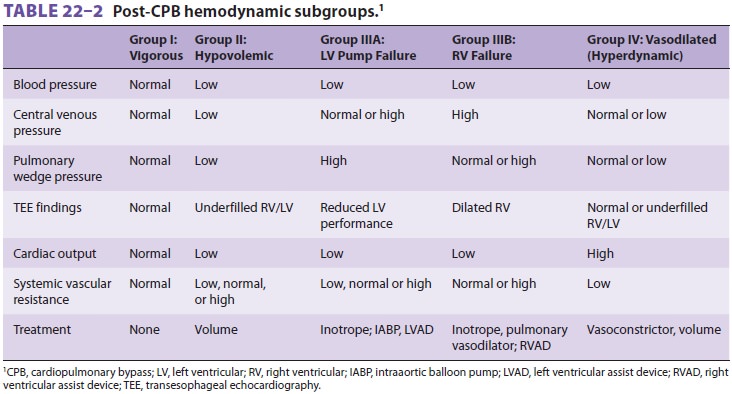
and the increase becomes progressively
more sus-tained. Most of these patients maintain good blood pressure and
cardiac output with a left ventricular filling pressure below 10–15 mm Hg.
Ventricular impairment should be suspected (when definitive diagnosis using TEE
is not available) in hypovolemic patients whose filling pressures rise during
volume infusion without appreciable changes in blood pres-sure or cardiac
output or in those who require filling pressures above 10–15 mm Hg. Ventricular
dysfunc-tion is easily diagnosed by TEE.
Patients with pump failure emerge from
CPB with a sluggish, poorly contracting heart that pro-gressively distends. In
such cases, CPB may need to be reinstituted while inotropic therapy is
initi-ated; alternatively, if the patient is less unstable, a positive inotrope
(epinephrine, dopamine, dobu-tamine) can be administered while the patient is
observed for improvement. If the patient does not respond to reasonable doses
of one of these three agents, milrinone can be added. In patients with poor
preoperative ventricular function milrinone may be administered as the
first-line agent prior to separation from CPB. In the rare instance that SVR is
increased, afterload reduction with nitroprus-side or milrinone can be tried.
The patient should be evaluated for unrecognized ischemia (kinked graft or
coronary vasospasm), valvular dysfunction, shunting, or right ventricular
failure (the distention is primarily right sided). TEE will facilitate the
diag-nosis in these cases.
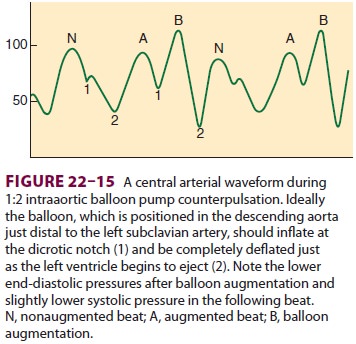
If drug therapies fail, intraaortic balloonpump (IABP)
counterpulsation should be initiatedwhile the patient is “rested” on CPB. The
efficacy of IABP depends on proper timing of inflation and deflation of the
balloon ( Figure
22–15). The bal-loon should
inflate just after the dicrotic notch is seen on the intraaortic pressure
tracing to aug-ment diastolic blood pressure and coronary flow after closure of
the aortic valve. Inflation too earlyincreases afterload and exacerbates
aortic regur-gitation, whereas late inflation reduces diastolic augmentation.
Balloon deflation should be timed just prior to left ventricular ejection to
decrease its afterload. Early deflation makes diastolic augmen-tation and
afterload reduction less effective. Use of a left or right ventricular assist
device (LVAD or
RVAD, respectively), may be necessary
for patients with refractory pump failure. If myocardial stun-ning is a major
contributor or there are areas of hibernating myocardium, a delayed improvement
in contractile function may allow complete wean-ing from all drugs and support
devices only after 12–48 h of therapy. Circulatory assist devices, such as the
Abiomed and HeartMate, can be used as a bridge to cardiac transplantation; the
former can be used for several days whereas the latter device can be left in place
for months to years.
Many clinicians believe that positive
inotropes should not routinely be used in patients coming off CPB because they
increase myocardial oxy-gen demand. The routine use of calcium similarly may
worsen ischemic injury and may contribute to coronary spasm (particularly in
patients who were taking calcium channel blockers preoperatively).
Nevertheless, there are centers that administer cal-cium salts or a positive
inotrope (eg, dobutamine), or both, to every patient at the conclusion of CPB.
Commonly used positive inotropes and vasopres-sors are listed in Table 22–3.
Epinephrine, dopa-mine, and dobutamine are the most commonly used agents.
Clinically, epinephrine is the most potent inotrope and is often effective in
increasing both cardiac output and systemic blood pressure when others agents
have failed. In lower doses, it
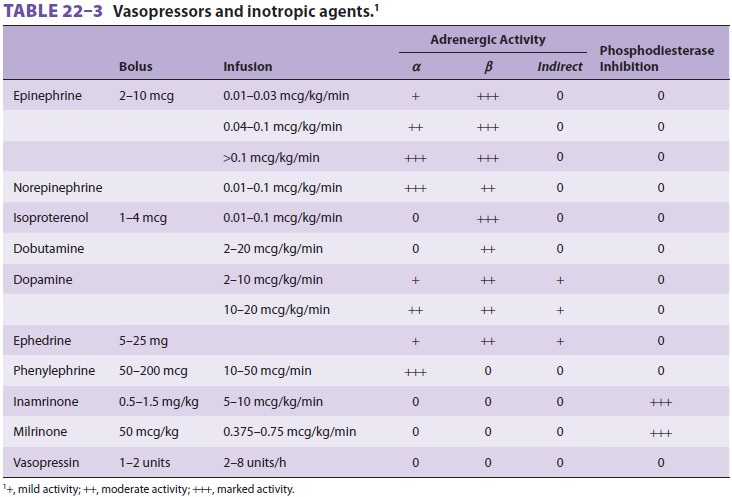
has predominantly β agonist
activity. Dobutamine, unlike dopamine, does not increase filling pres-sures and
may be associated with less
tachycardia than dopamine; unfortunately, cardiac output often increases
without significant changes in blood pres-sure. On the other hand, dopamine may
improve renal blood flow (at reduced doses) and is often more effective in
increasing blood pressure than in increasing cardiac output. Interestingly,
when infused to increase cardiac output to similar extents, epinephrine is
associated with no more increase (and perhaps less) in heart rate than
dobutamine. Inamrinone and milrinone, both selective phos-phodiesterase type
III inhibitors, are inotropes with arterial and venous dilator properties;
milrinone may be less likely than inamrinone to decrease the platelet count. In
studies of patients with chronic heart failure these two inodilators, unlike
other inotropes, did not appreciably increase myocardial oxygen consumption.
The combination of an ino-dilator (usually milrinone) and a β-adrenergic
agonist results in at least additive (and possibly synergistic) inotropic
effects. Norepinephrine is useful for increasing SVR but may compromise
splanchnic and renal blood flow at increased doses. Some clinicians use
norepinephrine in combina-tion with phosphodiesterase inhibitors to prevent
excessive reductions in systemic arterial pressure. Arginine vasopressin may be
used in patients with refractory hypotension, a low SVR, and resistance to
norepinephrine. Inhaled nitric oxide and pros-taglandin E1 may also be helpful for refractory pul-monary
hypertension and right ventricular failure (Table 22–4); nitric oxide has
the added advantage of not decreasing systemic arterial pressure. Studies have
not confirmed outcome benefits to the use of nesiritide, a human B-type
natriuretic peptide, thy-roid hormone (T 3),
or glucose–insulin–potassium
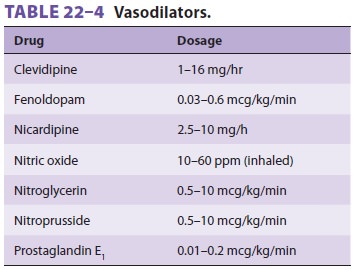
infusions for vasoactive/inotropic
support after CPB.
Related Topics