Chapter: Medical Surgical Nursing: Management of Patients With Coronary Vascular Disorders
Myocardial Infarction - Coronary Artery Disease
MYOCARDIAL INFARCTION
Pathophysiology
MI
refers to the process by which areas of myocardial cells in the heart are
permanently destroyed. Like unstable angina, MI is usually caused by reduced
blood flow in a coronary artery due to atherosclerosis and occlusion of an
artery by an embolus or thrombus. Because unstable angina and acute MI are
considered to be the same process but different points along a continuum, the
term acute coronary syndrome (ACS)
may be used for these di-agnoses. Other causes of an MI include vasospasm
(sudden con-striction or narrowing) of a coronary artery; decreased oxygen
supply (eg, from acute blood loss, anemia, or low blood pressure); and
increased demand for oxygen (eg, from a rapid heart rate, thy-rotoxicosis, or
ingestion of cocaine). In each case, a profound im-balance exists between
myocardial oxygen supply and demand.
Coronary
occlusion, heart attack, and MI are terms used syn-onymously, but the preferred
term is MI. The area of infarction takes time to develop. As the cells are
deprived of oxygen, ischemia develops, cellular injury occurs, and over time,
the lack of oxygen results in infarction, or the death of cells. The expression
“time is muscle” reflects the urgency of appropriate treatment to improve
patient outcomes. Each year in the United States, nearly 1 million people have
acute MIs; one fourth of these people die of MI (Amer-ican Heart Association,
2001; Ryan et al., 1999). One half of those who die never reach a hospital.
Various
descriptions are used to further identify an MI: the lo-cation of the injury to
the left ventricular wall (anterior, inferior, posterior, or lateral wall) or
to the right ventricle and the point in time within the process of infarction
(acute, evolving, or old).
The
ECG usually identifies the location, and the ECG and pa-tient history identify
the timing. Regardless of the location of the infarction of cardiac muscle, the
goal of medical therapy is to pre-vent or minimize myocardial tissue death and
to prevent compli-cations.
Clinical Manifestations
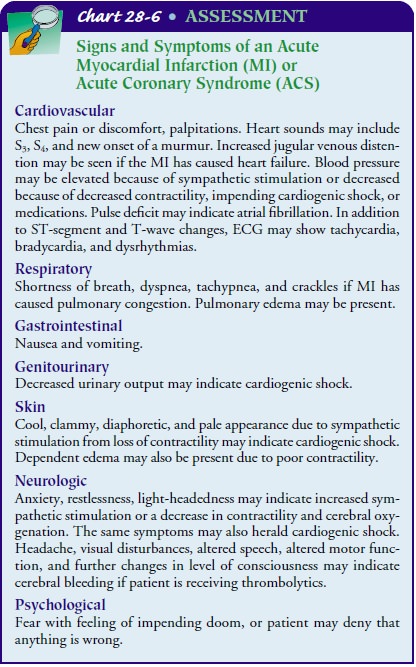
Chest pain that occurs suddenly and continues despite rest and medication is the presenting symptom in most patients with an MI (Chart 28-6). One study showed that 2% of patients who eventually were diagnosed with an acute MI were incorrectly dis-charged and sent home from the emergency department (Pope et al., 2000). Most of these patients presented with atypical symp-toms such as shortness of breath; they also tended to be female, younger than 55 years of age, of a minority group, and have nor-mal ECGs. The Framingham Heart Study revealed that 50% of the men and 63% of the women who died suddenly of cardio-vascular disease had no previous symptoms (Kannel, 1986). Pa-tients may also be anxious and restless. They may have cool, pale, and moist skin. Their heart rate and respiratory rate may be faster than normal. These signs and symptoms, which are caused by stimulation of the sympathetic nervous system, may be present only for a short time or may not be present, or only some of them may occur. In many cases, the signs and symptoms of MI cannot be distinguished from those of unstable angina.
Assessment and Diagnostic Findings
Diagnosis
of MI is generally based on the presenting symptoms, the ECG, and laboratory
test results (eg, serial serum enzyme values). The prognosis depends on the
severity of coronary artery obstruction and the extent of myocardial damage.
Phys-ical examination is always conducted, but the examination alone is
insufficient to confirm the diagnosis.
PATIENT HISTORY
The
patient history has two parts: the description of the pre-senting symptom (eg,
pain) and the history of previous illnesses and family health history,
particularly of heart disease. Previous history should also include information
about the patient’s risk factors for heart disease.
ELECTROCARDIOGRAM
The
ECG provides information that assists in diagnosing acute MI. It should be
obtained within 10 minutes from the time a patient reports pain or arrives in
the emergency department. By monitor-ing the ECG over time, the location,
evolution, and resolution of an MI can be identified and monitored.
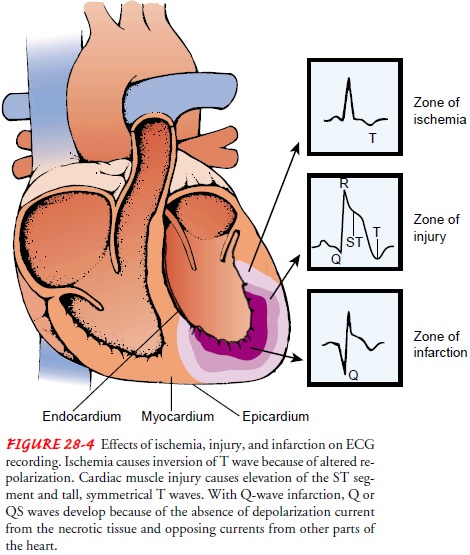
The
ECG changes that occur with an MI are seen in the leads that view the involved
surface of the heart. The classic ECG changes are T-wave inversion, ST-segment
elevation, and development of an abnormal Q wave (Fig. 28-4). Because
infarction evolves over time, the ECG also changes over time. The first ECG
signs of an acute MI are from myocardial ischemia and injury. Myocardial injury
causes the T wave to become enlarged and symmetric. As the area of injury
becomes ischemic, myocardial repolarization is altered and delayed, causing the
T wave to invert. The ischemic region may remain depolarized while adjacent
areas of the myo-cardium return to the resting state. Myocardial injury also
causes ST-segment changes. The injured myocardial cells depolarize nor-mally
but repolarize more rapidly than normal cells, causing the ST segment to rise
at least 1 mm above the isoelectric line (area between the T wave and the next
P wave is used as the reference for the isoelectric line) when measured 0.08
seconds after the end of the QRS. If the myocardial injury is on the
endocardial surface, the ST segment is depressed 1 mm or more for at least 0.08
seconds. The ST-segment depression is usually horizontal or has a downward
slope (Wagner, 2001).
MI
is classified as a Q-wave or non-Q-wave infarction. With Q-wave infarction,
abnormal Q waves develop within 1 to 3 days because there is no depolarization
current conducted from necrotic tissue (Wagner, 2001). The lead system then
views the flow of current from other parts of the heart. An abnormal Q wave is
0.04 seconds or longer, 25% of the R-wave depth (pro-vided the R wave exceeds a
depth of 5 mm), or one that did not exist before the event (Wagner, 2001). An
acute MI may cause a significant decrease in the height of the R wave. During
an acute MI, injury and ischemic changes are also present. An abnormal Q wave
may be present without ST-segment and T-wave changes, which indicates an old,
not acute, MI. Patients with non-Q-wave MIs do not develop a Q wave on the ECG
after the ST-segment and T-wave changes, but symptoms and cardiac enzyme
analysis confirm the diagnosis of an MI.
During
recovery from an MI, the ST segment often is the first to return to normal (1
to 6 weeks). The T wave becomes large and symmetric for 24 hours, and it then
inverts within 1 to 3 days for 1 to 2 weeks. Q-wave alterations are usually
permanent. An old Q-wave MI is usually indicated by an abnormal Q wave or
decreased height of the R wave without ST-segment and T-wave changes.
ECHOCARDIOGRAM
The
echocardiogram is used to evaluate ventricular function. It may be used to
assist in diagnosing an MI, especially when the ECG is nondiagnostic. The
echocardiogram can detect hypo-kinetic and akinetic wall motion and can
determine the ejection fraction.
LABORATORY TESTS
Historically,
laboratory tests used to diagnose an MI included creatine kinase (CK), with evaluation of isoenzymes and
lacticdehydrogenase (LDH) levels. Newer laboratory tests with faster results,
resulting in earlier diagnosis, include myoglobin and tro-ponin analysis. These
tests are based on the release of cellular con-tents into the circulation when
myocardial cells die. Table 28-5 shows the time courses of cardiac enzymes. An
LDH test is now infrequently ordered because it is not useful in identifying
cardiac events (Braunwald et al., 2000).
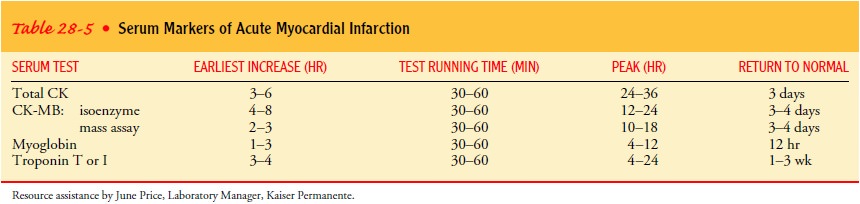
Creatine Kinase and Its Isoenzymes.
There are three CKisoenzymes: CK-MM (skeletal muscle), CK-MB
(heart muscle), and CK-BB (brain tissue). CK-MB is the cardiac-specific
iso-enzyme; CK-MB is found mainly in cardiac cells and therefore rises only
when there has been damage to these cells. CK-MB assessed by mass assay is the
most specific index for the diagnosis of acute MI (Braunwald et al., 2001). The
level starts to increase within a few hours and peaks within 24 hours of an MI.
If the area is reper-fused (eg, due to thrombolytic therapy or PTCA), it peaks
earlier.
Myoglobin.
Myoglobin is a heme protein that helps to transportoxygen. Like CK-MB enzyme, myoglobin is found in cardiac and skeletal muscle. The myoglobin level starts to increase within 1 to 3 hours and peaks within 12 hours after the onset of symptoms.
The
test takes only a few minutes to run. An increase in myoglobin is not very
specific in indicating an acute cardiac event; however, negative results are an
excellent parameter for ruling out an acute MI. If the first myoglobin test
results are negative, the test may be repeated 3 hours later. Another negative
test result confirms that the patient did not have an MI.
Troponin.
Troponin,
a protein found in the myocardium, regu-lates the myocardial contractile
process. There are three isomers of troponin (C, I, and T). Because of the
smaller size of this protein and the increased specificity of the troponins I
and T for cardiac muscle, these tests are used more frequently to identify
myocardial injury (unstable angina or acute MI). The increase in the level of
troponin in the serum starts and peaks at approximately the same time as CK-MB.
However, it remains elevated for a longer period, often up to 3 weeks, and it
therefore cannot be used to identify sub-sequent extension or expansion of an
MI.
Medical Management
The
goal of medical management is to minimize myocardial dam-age, preserve
myocardial function, and prevent complications. These goals are achieved by
reperfusing the area with the emer-gency use of thrombolytic medications or
PTCA. Minimizing my-ocardial damage is also accomplished by reducing myocardial
oxygen demand and increasing oxygen supply with medications, oxygen
administration, and bed rest. The resolution of pain and ECG changes are the
primary clinical indicators that demand and supply are in equilibrium; they may
also indicate reperfu-sion. Visualization of blood flow through an open vessel
in the catheterization laboratory is evidence of reperfusion.
PHARMACOLOGIC THERAPY
The
patient with an acute MI receives the same medications as the patient with
unstable angina, with the possible additions of thrombolytics, analgesics, and
angiotensin-converting enzyme (ACE) inhibitors. Patients should receive a
beta-blocker initially, throughout the hospitalization, and a prescription to
continue its use after hospital discharge.
Thrombolytics.
Thrombolytics
are medications that are usually ad-ministered intravenously, although some may
also be given directly into the coronary artery in the cardiac catheterization
laboratory (Chart 28-7). The purpose of thrombolytics is to dissolve and lyse
the thrombus in a coronary artery (thrombolysis), allowing blood to flow
through the coronary artery again (reperfusion), minimiz-ing the size of the
infarction, and preserving ventricular function. Even though thrombolytics may
dissolve the thrombus, they do not affect the underlying atherosclerotic
lesion. The patient may be referred for a cardiac catheterization and other
invasive inter-ventions.
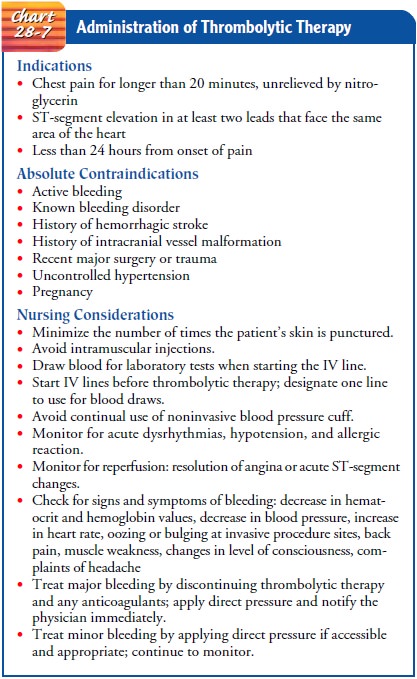
Thrombolytics dissolve all clots, not just the one in the coro-nary artery. They should not be used if the patient has formed a protective clot, such as after major surgery or hemorrhagic stroke. Because thrombolytics reduce the patient’s ability to form a stabi-lizing clot, the patient is at risk for bleeding. Thrombolytics should not be used if the patient is bleeding or has a bleeding disorder. All patients who receive thrombolytic therapy are placed on bleeding precautions to minimize the risk for bleeding. This means mini-mizing the number of punctures for inserting intravenous lines, avoiding intramuscular injections, preventing tissue trauma, and applying pressure for longer than usual after any puncture.
To
be effective, thrombolytics must be administered as early as possible after the
onset of symptoms that indicate an acute MI. They are not given to patients
with unstable angina. Hospitals mon-itor their ability to administer these
medications within 30 minutes from the time the patient arrives in the
emergency department. This is called door-to-needle
time (Ryan et al., 1999). The thrombolytic agents used most often are streptokinase (Kabikinase, Streptase),
alteplase (Activase), and reteplase (r-PA, TNKase). Anistreplase (Eminase) is
another thrombolytic agent that may be used.
Streptokinase
increases the amount of plasminogen activator, which then increases the amount
of circulating and clot-bound plasmin. Because streptokinase is made from a
bacterium, its use also entails a risk of an allergic reaction. Vasculitis has
occurred up to 9 days after administration. Streptokinase is not used if the
patient has been exposed to a recent Streptococcus
infection or has received streptokinase in the past 6 to 12 months.
Alteplase
is a type of tissue plasminogen activator (t-PA). In contrast to streptokinase,
alteplase activates the plasminogen on the clot more than the circulating
plasminogen. Because it does not decrease the clotting factors as much as
streptokinase, un-fractionated or low molecular weight heparin is used with
t-PA to prevent another clot from forming at the same lesion site. Because t-PA
is a naturally occurring enzyme, allergic reactions are mini-mized, but t-PA
costs considerably more than streptokinase.
Reteplase
is structurally very similar to alteplase and has similar effects. Anistreplase
is similar to streptokinase and has similar effects.
Analgesics.
The
analgesic of choice for acute MI is morphinesulfate (Duramorph, Astramorph)
administered in intravenous boluses. Morphine reduces pain and anxiety. It
reduces preload, which decreases the workload of the heart. Morphine also
relaxes bronchioles to enhance oxygenation. The cardiovascular response to
morphine is monitored carefully, particularly the blood pres-sure, which can be
lowered, and the respiratory rate, which can be depressed. Because morphine
decreases sensation of pain, ST-segment monitoring may be a better indicator of
subsequent ischemia than assessment of pain.
Angiotensin-Converting Enzyme Inhibitors (ACE-I).
Angiotensin Iis formed when the kidneys release
renin in response to decreased blood flow. Angiotensin I is converted to
angiotensin II by ACE, a substance found in the lumen of all blood vessels,
especially the pulmonary vasculature. Angiotensin II causes the blood vessels
to constrict and the kidneys to retain sodium and fluid while excreting
potassium. These actions increase circulating fluid and raise the pressure
against which the heart must pump, result-ing in significantly increased
cardiac workload. ACE inhibitors(ACE-I) prevent
the conversion of angiotensin from I to II. Inthe absence of angiotensin II,
the blood pressure decreases and the kidneys excrete sodium and fluid
(diuresis), decreasing the oxygen demand of the heart. Use of ACE inhibitors in
patients after MI decreases the mortality rate and prevents the onset of heart
failure. It is important to ensure that the patient is not hypotensive,
hyponatremic, hypovolemic, or hyperkalemic before ACE-I ad-ministration. Blood
pressure, urine output, and serum sodium, potassium, and creatinine levels need
to be monitored closely.
EMERGENT PERCUTANEOUS CORONARY INTERVENTION (PCI)
The
patient in whom an acute MI is suspected may be referred for an immediate PCI.
PCI may be used to open the occluded coro-nary artery in an acute MI and
promote reperfusion to the area that has been deprived of oxygen. PCI treats
the underlying atheroscle-rotic lesion. Because the duration of oxygen
deprivation is directly related to the number of cells that die, the time from
the patient’s arrival in the emergency department to the time PCI is performed
should be less than 60 minutes (time is muscle). This is frequently referred to
as door-to-balloon time (Smith et
al., 2001). To perform an emergent PCI within this short time, a cardiac
catheterization laboratory and staff must be available.
Cardiac Rehabilitation
After
the MI patient is free of symptoms, an active rehabilitation program is
initiated. Cardiac rehabilitation is a program that tar-gets risk reduction by
means of education, individual and group support, and physical activity. Most
insurance programs, in-cluding Medicare, cover the cost of a cardiac
rehabilitation pro-gram. However, some studies indicate that only 8% to 39% of
patients who are candidates for cardiac rehabilitation services typically
participate in these programs (Wenger et al., 1995; Williams et al., 2002).
The
goals of rehabilitation for the patient with an MI are to ex-tend and improve
the quality of life. The immediate objectives are to limit the effects and
progression of atherosclerosis, return the pa-tient to work and a pre-illness
lifestyle, enhance the psychosocial and vocational status of the patient, and
prevent another cardiac event. These objectives are accomplished by encouraging
physical activity and physical conditioning, educating patient and family, and
providing counseling and behavioral interventions.
Throughout
all phases of rehabilitation, the goals of activity and exercise tolerance are
achieved through gradual physical condi-tioning, aimed at improving cardiac
efficiency over time. Cardiac efficiency is achieved when work and activities
of daily living can be performed at a lower heart rate and lower blood
pressure, thereby reducing the heart’s oxygen requirements and reducing cardiac
workload.
Physical
conditioning is achieved gradually over time. It is not unusual for patients to
“overdo it” in an attempt to achieve their goals too rapidly. Patients are
observed for chest pain, dyspnea, weakness, fatigue, and palpitations and are
instructed to stop exer-cise if any of the symptoms develop. In a monitored
program, they are also monitored for an increase in heart rate above the target
heart rate, an increase in systolic or diastolic blood pressure more than 20 mm
Hg, a decrease in systolic blood pressure, onset or worsening of dysrhythmias,
or ST-segment changes on the ECG.
The
target heart rate in phase I is an increase of less than 10% from the resting
heart rate, or 120 beats per minute. In phase II, the target heart rate is
based on the results of the patient’s stress test (usually 60% to 85% of the
heart rate at which symptoms oc-curred), medications, and underlying condition.
Oxygen satura-tion may also be assessed to ensure that it remains higher than
93%. If signs or symptoms occur, the patient is instructed to slow down or stop
exercising. If the patient is exercising in an un-monitored program, he or she
is cautioned to cease activity im-mediately if signs or symptoms occur and to seek
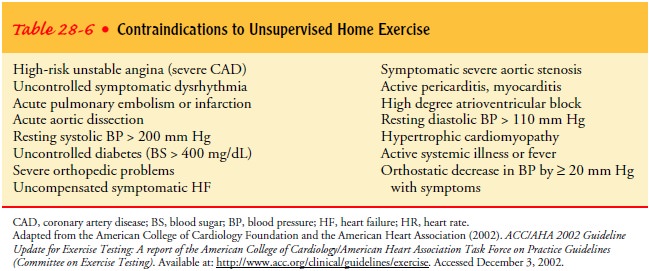
appropriate medical attention. Table 28-6 identifies conditions in which an
unmonitored home exercise program is not recommended.
Patients
who are able to walk at 3 to 4 miles per hour are usually able to resume sexual
activities. The nurse recommends that the patient be well rested and in a
familiar setting; wait at least 1 hour after eating or drinking alcohol; and
use a comfortable position. The patient is cautioned against anal sex. Sexual
dysfunction or cardiac symptoms should be reported to the health care provider.
PHASES OF CARDIAC REHABILITATION
Cardiac rehabilitation occurs along the continuum of the disease and is typically categorized in three phases. Phase I may begin with the diagnosis of atherosclerosis, which may occur when the patient is admitted to the hospital for ACS (unstable angina, acute MI). It consists of low-level activities and initial education for the patient and family.
Because
of the brief hospital stay, mo-bilization occurs earlier, and patient teaching
is prioritized to the essentials of self-care, rather than instituting
behavioral changes for risk reduction. Priorities for in-hospital education
include the signs and symptoms that indicate the need to call 911 (seek
emer-gency assistance), the medication regimen, rest-activity balance, and
follow-up appointments with the physician. The nurse needs to reassure the
patient that, although CAD is a lifelong disease and must be treated as such,
most patients can resume a normal life after an MI. This positive approach
while in the hospital helps to motivate and teach the patient to continue the
education and lifestyle changes that are usually needed after discharge. The
amount of activity recommended at discharge depends on the age of the patient,
his or her condition before the cardiac event, the extent of the disease, the
course of the hospital stay, and the development of any complications.
Phase
II occurs after the patient has been discharged. It usu-ally lasts for 4 to 6
weeks but may last up to 6 months. This out-patient program consists of
supervised, often ECG-monitored, exercise training that is individualized based
on the results of an exercise stress test. Support and guidance related to the
treatment of the disease and education and counseling related to lifestyle
modification for risk factor reduction are a significant part of this phase.
Short-term and long-range goals are collaboratively deter-mined based on the
patient’s needs. At each session, the patient is assessed for the effectiveness
of and adherence to the current medical plan. To prevent complications and
another hospitaliza-tion, the cardiac rehabilitation staff alerts the referring
physician to any problems. Outpatient cardiac rehabilitation programs are
designed to encourage patients and families to support each other. Many
programs offer support sessions for spouses and sig-nificant others while the
patients exercise. The programs involve group educational sessions for both
patients and families that are given by cardiologists, exercise physiologists,
dietitians, nurses, and other health care professionals. These sessions may
take place outside a traditional classroom setting. For instance, a dietitian
may take a group of patients and their families to a grocery store to examine
labels and meat selections or to a restaurant to discuss menu offerings for a
“heart-healthy” diet.
Phase
III focuses on maintaining cardiovascular stability and long-term conditioning.
The patient is usually self-directed during this phase and does not require a
supervised program, although it may be offered. The goals of each phase build
on the accomplish-ments of the previous phase.
Related Topics