Chapter: Medical Surgical Nursing: Management of Patients With Coronary Vascular Disorders
Invasive Interventional Procedures - Invasive Coronary Artery Procedures
Invasive Coronary Artery Procedures
INVASIVE INTERVENTIONAL PROCEDURES
Angina
pectoris may persist for many years in a stable form with brief attacks.
However, unstable angina is a serious condition that can progress to MI or
sudden cardiac death (ACS). Invasive in-terventional procedures to treat angina
and CAD are PTCA, in-tracoronary stent implantation, atherectomy,
brachytherapy, and transmyocardial laser revascularization. All of these
procedures are classified as percutaneous
coronary interventions (PCIs).
Percutaneous Transluminal Coronary Angioplasty (PTCA)
PTCA
may be used to treat patients who do not experience angina but are at high risk
for a cardiac event as identified by non-invasive testing, with recurrent chest
pain that is unresponsive to medical therapy, with a significant amount of
myocardium at risk but are poor surgical candidates, or with an acute MI (as an
al-ternate to thrombolysis and after thrombolysis) (Smith et al., 2001). The
procedure is attempted when the cardiologist believes that PTCA can improve
blood flow to the myocardium. PTCA alone is seldom attempted in the patient
with occlusions of the left main coronary artery that do not demonstrate collateral cir-culation to the left
anterior descending and circumflex arteries.The purpose of PTCA is to improve
blood flow within a coro-nary artery by “cracking” the atheroma.
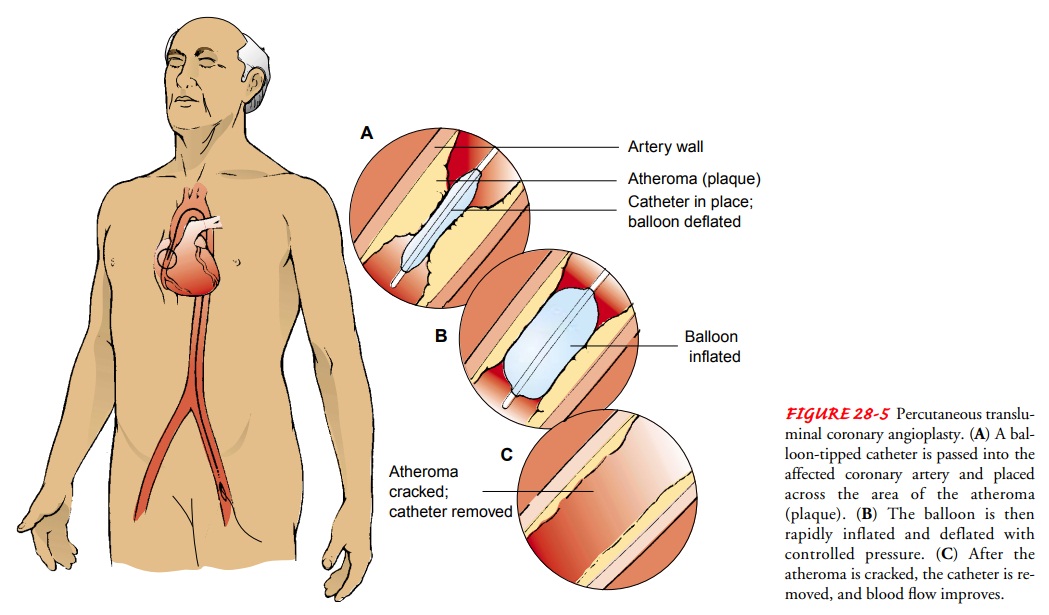
This invasive interventional procedure is carried out in the cardiac catheterization laboratory. The coronary arteries are ex-amined by angiography, as they are during the diagnostic cardiac catheterization, and the location, extent, and calcification of the atheroma are verified. Hollow catheters, called sheaths, are in-serted, usually in the femoral vein or artery (or both), providing a conduit for other catheters. After the presence of atheroma is verified, a balloon-tipped dilation catheter is passed through the sheath along a guide catheter and positioned over the lesion. The physician determines the catheter position by examining mark-ers on the balloon that can be seen with fluoroscopy. When the catheter is properly positioned, the balloon is inflated with a radiopaque contrast agent (commonly called dye) to visualize the blood vessel and to provide a steady or oscillating pressure within the balloon.
The balloon is inflated to a certain pressure for sev-eral seconds and then deflated. The pressure “cracks” and possi-bly compresses the atheroma (Fig. 28-5). The coronary artery’s media and adventitia are also stretched.Several inflations and several balloon sizes may be required to achieve the desired goal, usually defined as an improvement in blood flow and a residual stenosis of less than 20%. Other gauges of the success of a PTCA are an increase in the artery’s lumen, a difference of less than 20 mm Hg in blood pressure from one side of the lesion to the other, and no clinically obvious arterial trauma.
Because the
blood supply to the coronary artery decreases while the balloon is inflated,
the patient may complain of chest pain (often called stretch pain), and the ECG may display signif-icant ST-segment
changes ( Jeremias et al., 1998).
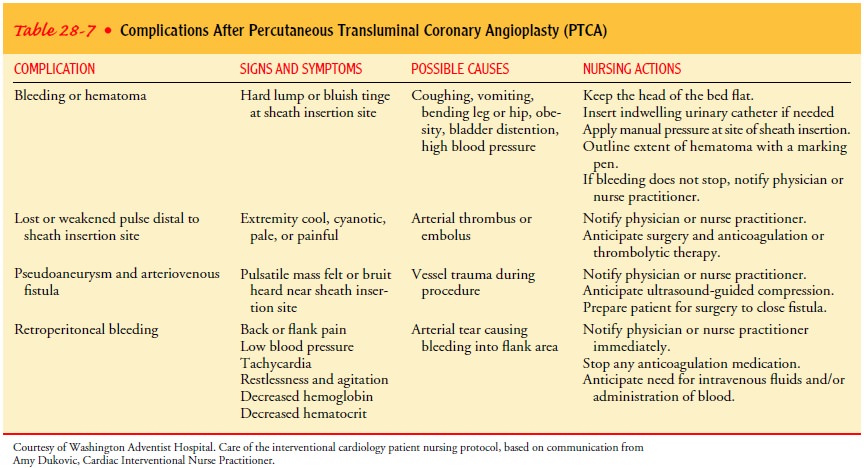
COMPLICATIONS
Possible
complications during the PTCA procedure include dis-section, perforation,
abrupt closure, or vasospasm of the coronary artery, acute MI, acute
dysrhythmias (eg, ventricular tachycardia), and cardiac arrest. These may
require emergency surgical treat-ment. Complications after the procedure may
include abrupt clo-sure and vascular complications, such as bleeding at the
insertion site, retroperitoneal bleeding, hematoma, pseudoaneurysm,
arte-riovenous fistula, or arterial thrombosis and distal embolization (Table
28-7).
POSTPROCEDURE CARE
Patient
care is similar to that for a cardiac catheterization. Many patients are
admitted to the hospital the day of the PTCA. Those with no complications go
home the next day. During the PTCA, patients receive large amounts of heparin
and are monitored closely for signs of bleeding. Most patients also receive
intravenous nitroglycerin for a period after the procedure to prevent arterial
spasm.
Hemostasis
is usually achieved and sheaths are pulled imme-diately at the end of the
procedure by using a vascular closure de-vice (eg, Angio-Seal, VasoSeal, Duett,
Syvek patch) or a device that sutures the vessels (Prostar, Perclose).
Hemostasis after sheath removal may also be achieved by direct manual pressure,
a mechanical compression device (eg, C-shaped clamp), or a pneumatic
compression device (eg, FemStop).
The
patient may return to the nursing unit with the large pe-ripheral vascular
access sheaths in place. The sheaths are removed after blood studies (eg,
activated clotting time) indicate that the clotting time is within an
acceptable range. This usually takes a few hours, depending on the amount of
heparin given during the pro-cedure. The patient must remain flat in bed and
keep the affected leg straight until the sheaths are removed and then for a few
hours after to maintain hemostasis. Because the immobility and bed rest usually
cause the patient significant discomfort, treatment includes analgesics and
sedation.
Several
nursing interventions frequently used as part of the standard of care, such as
applying a sandbag to the sheath inser-tion site, have not been shown to be
effective in reducing the in-cidence of bleeding (Christensen et al., 1998;
Juran et al., 1999). The method used to achieve hemostasis determines the
length of time needed to achieve hemostasis, the duration of bed rest, and the
risk of complications (Brachmann et al., 1998; Lehmann et al., 1999; Walker et
al., 2001). Sheath removal and the appli-cation of pressure on the vessel
insertion site may cause the heart rate to slow and the blood pressure to
decrease (vasovagal response). An intravenous bolus of atropine is usually used
to treat these side effects.
Some
patients with unstable lesions and at high risk for abrupt vessel closure are
restarted on heparin after sheath removal, or they receive an intravenous
infusion of a GPIIb/IIIa inhibitor. These patients are monitored more closely
and progressed more slowly.
After hemostasis is achieved, patients usually can be weaned from the intravenous medications, resume self-care, and ambulate unassisted within 1 to 12 hours of the procedure. The duration of immobilization depends on the size of the sheath inserted, the amount of anticoagulant administered, the method of hemostasis, the patient’s underlying condition, and the physician’s preference. The nurse teaches the patient to monitor the site for bleeding or development of a hard lump that is larger than a walnut. Most patients can return to their usual activities of daily living.
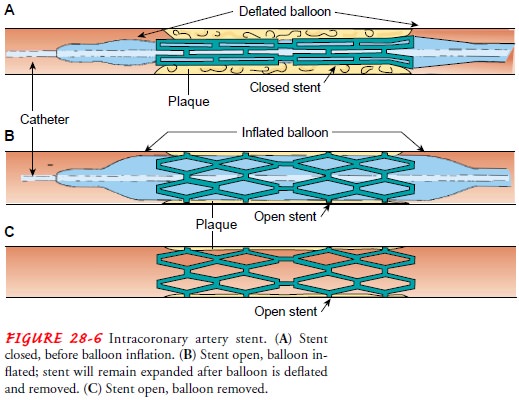
Coronary Artery Stent
After
PTCA, a portion of the plaque that was not removed may block the artery. The
coronary artery may recoil (constrict) and the tissue remodels, increasing the
risk for restenosis (Apple & Lindsay, 2000). A coronary artery stent is
placed to overcome these risks. A stent
is a woven mesh that provides structural support to a vessel at risk of acute
closure. The stent is placed over the angioplasty balloon. When the balloon is
inflated, the mesh expands and presses against the vessel wall, holding the
artery open. The balloon is withdrawn, but the stent is left permanently in
place within the artery (Fig. 28-6). Eventually, endothelium covers the stent
and it is incorporated into the vessel wall. Because of the risk of thrombus
formation in the stent, the patient receives antiplatelet medications (eg,
clopidogrel [Plavix] therapy for 2 weeks and lifetime use of aspirin). Some
stents have medication which may minimize the formation of thrombi or excessive
scar tissue. It is estimated that 50% to 80% of all PCIs in-volve implanting at
least one stent (Braunwald et al., 2001; Smith et al., 2001). Stents may be
used in conjunction with PTCA or inde-pendently as a PCI. Use of stents without
PTCA may decrease pro-cedure time, use of the potentially nephrotoxic contrast
agent, radiation exposure, and cost (Apple & Lindsay, 2000). Care of the
patient after coronary artery stent placement is the same as for a pa-tient
after PTCA.
Atherectomy
Atherectomy
is an invasive interventional procedure that involves the removal of the
atheroma, or plaque, from a coronary artery (Smith et al., 2001). Directional
(DCA) and transluminal ex-traction (TEC) coronary atherectomy procedures
involve the use of a catheter that removes the lesion and its fragments.
Rotational atherectomy uses a catheter with diamond chips impregnated on the
tip (called a burr) that rotates like a dentist’s drill at 130,000 to 180,000
rpm, pulverizing the lesion (Braunwald et al., 2001). Usually, several passes
of these catheters are needed to achieve sat-isfactory results. Postprocedural
patient care is the same as for a patient after PTCA.
Brachytherapy
PTCA
and stent implantation cause a cellular reaction in the coronary artery that
promotes proliferation of the intima of the artery, which also increases the
possibility of arterial obstruction. Brachytherapy reduces the recurrence of
obstruction, preventing vessel restenosis by inhibiting smooth muscle cell
proliferation (Leon et al., 2001). Brachytherapy (from the Greek word, brachys, meaning short) involves the delivery of gamma or beta radiation by placing
a radioisotope close to the lesion (Teirstein & Kuntz, 2001). The
radioisotope may be delivered by a catheter or im-planted with the stent. Long-term
studies are needed to identify if the beneficial effects of radiation therapy
are sustained and to determine the optimal dose and type of isotope to use for
brachytherapy.
Transmyocardial Revascularization
Patients who have cardiac ischemia and who are not candidates for CABG may benefit from transmyocardial laser revasculariza-tion (TMR) (Burkhoff et al., 1999). The procedure may be per-formed percutaneously in the cardiac catheterization laboratory (percutaneous transmyocardial revascularization [PTMR]) or through a midsternal or thoracotomy incision in the operating room (Acorda et al., 2000). The tip of a fiberoptic catheter is held firmly against the ischemic area of the heart while a laser burns a channel into but not through the muscle. If the proce-dure is percutaneous, the catheter is positioned inside the ven-tricle. If the procedure is surgical, the catheter is positioned on the outer surface of the ventricle. Each procedure usually in-volves making 20 to 40 channels. It is thought that some blood flows into the channels, decreasing the ischemia directly. Within the next few days to months, the channels close as a result of the body’s inflammatory process of healing a wound (Platek & Atzori, 1999). The long-term result is the formation of new blood ves-sels (angiogenesis) during the inflammatory process that fol-lows the laser burns (Anderson, 2000; Braunwald et al., 2001; Fuster et al., 2001; Hayden, 1998; Platek, & Atzori, 1999). The new blood vessels provide enough blood to decrease the symp-toms of cardiac ischemia. Nursing care before, during, and after the procedure depends on the approach: if the approach was per-cutaneous, the patient care is the same as following a PTCA; if the approach was surgical, the patient care is the same as follow-ing CABG.
Related Topics