with Solved Example | Chemical Kinetics - Molecularity | 12th Chemistry : UNIT 7 : Chemical Kinetics
Chapter: 12th Chemistry : UNIT 7 : Chemical Kinetics
Molecularity
Molecularity:
Kinetic studies involve
not only measurement of a rate of reaction but also proposal of a reasonable
reaction mechanism. Each and every single step in a reaction mechanism is
called an elementary reaction.
An elementary step is
characterized by its molecularity. The total number of reactant species that
are involved in an elementary step is called molecularity of that particular
step. Let us recall the hydrolysis of t-butyl bromide studied in XI standard.
Since the rate determining elementary step involves only t-butyl bromide, the
reaction is called a Unimolecular Nucleophilic substitution (SN1
) reaction.
Let us understand the
elementary reactions by considering another reaction, the decomposition of
hydrogen peroxide catalysed by I−.
2H2 O2
(aq) → 2H2O(l) + O2 (g)
It is experimentally
found that the reaction is first order with respect to both H2 O2
and I−,which indicates that I− is also involved in the
reaction. The mechanism involves the following steps.
Step:1
H2 O2
(aq) + I− (aq) → H2O(l) + OI−(aq)
Step : 2
H2O2 (aq) + OI− (aq) → H2O(l)
+ I−(aq) + O2 (g)
Overall reaction is
2H2O2
(aq) → 2H2O(l) + O2 (g)
These two reactions are
elementary reactions. Adding equ (1) and (2) gives the overall reaction. Step 1
is the rate determining step, since it involves both H2O2
and I−, the overall reaction
is bimolecular.
Differences between order and molecularity:
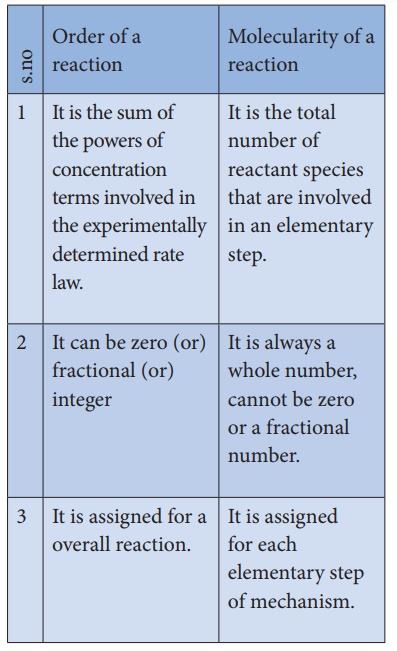
Order of a reaction
1. It is the sum of the powers of
concentration terms involved in the experimentally determined rate law.
2. It can be zero (or) fractional
(or) integer
3. It is assigned for a overall
reaction.
Molecularity of a reaction
1. It is the total number of
reactant species that are involved in an elementary step.
2. It is always a whole number, cannot
be zero or a fractional number.
3. It is assigned for each
elementary step of mechanism.
Example
1
Consider the oxidation of nitric
oxide to form NO2
2NO(g) + O2 (g) → 2NO2
(g)
(a). Express the rate of the
reaction in terms of changes in the concentration of NO,O2 and NO2
.
(b). At a particular instant, when
[O2] is decreasing at 0.2 mol L−1s−1 at what
rate is [NO2 ] increasing at that instant?
Solution:
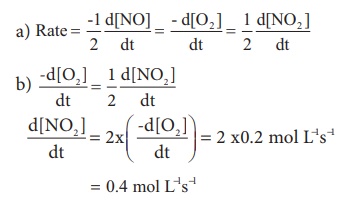
Evaluate
yourself 1
1) Write the rate expression for the
following reactions, assuming them as elementary reactions.
i) 3A + 5B2 →4CD
ii) X2 + Y2
→2XY
2). Consider the decomposition of N2O5
(g) to form NO2 (g) and O2 (g) . At a particular instant
N2O5 disappears at a rate of 2.5x10-2 mol dm-3s-1
. At what rates are NO2 and O2 formed? What is the rate
of the reaction?
Example
2
1. What is the order with respect to
each of the reactant and overall order of the following reactions?
(a). 5Br− ( aq ) + BrO3−(aq
) + 6H+ (aq)
→ 3Br2 (l ) + 3H2O(l)
The experimental rate law is
Rate = k [Br− ][BrO3][H+]2
(b). CH3CHO(g ) →Δ→
CH4 (g) + CO(g)
the experimental rate law is
Rate = k [CH3CHO]3/2
Solution:
a) First order with respect to Br−,
first order with respect to BrO3− and second order with
respect to H+ . Hence the overall order of the reaction is equal to 1 + 1 + 2 =
4
b) Order of the reaction with
respect to acetaldehyde is 3/2 and overall order is also 3/2
Example
3
2. The rate of the reaction x + 2y →
product is 4 x 10−3 mol L−1s−1 if [x]=[y]=0.2
M and rate constant at 400K is 2 x 10-2 s-1 , What is the
overall order of the reaction.
Solution
:
Rate = k [x]n [y]m
4 x 10-3 mol L-1s-1
= 2 x 10-2 s-1 (0.2 mol L-1 )n
(0.2mol L-1 )m
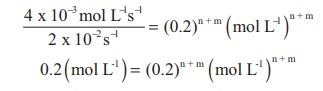
Comparing the powers on both sides
The overall order of the reaction n
+ m = 1
Evaluate
yourself 2
1). For a reaction, X + Y → product
; quadrupling [x] , increases the rate by a factor of 8. Quadrupling both [x]
and [y], increases the rate by a factor of 16. Find the order of the reaction
with respect to x and y. what is the overall order of the reaction?
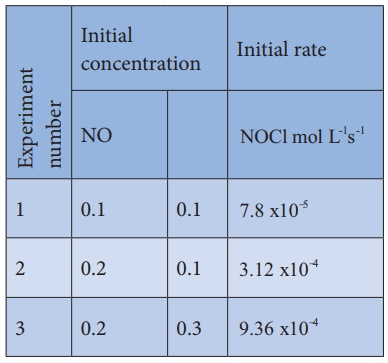
2). Find the individual and overall
order of the following reaction using the given data.
2NO(g) + Cl2 (g) →
2NOCl(g)
Related Topics