Chapter: Human Nervous System and Sensory Organs : Basic Elements of the Nervous System
Methods in Neuroanatomy - The Nerve Cell
Methods in Neuroanatomy
The
availability of methods for studying the structure and function of cells,
tissues, and organs is often the limiting factor in expanding our knowledge.
Certain terms and interpretations can only be understood if the background of
the method used is known. Therefore, the methods commonly used in neuroanatomy
are presented here briefly.
Nerve
cells and glial cells can be demon-strated in thin histological sections by
various histological techniques. The Nisslmethod
has proven helpful because of excel-lent visualization of the rough endoplasmicreticulum (p. 18),
which is abundant innerve cells. However, the different types of nerve cells
are essentially characterized by their long processes, the dendrites and the
axon, which are not stained by the Nissl method. For demonstration of as many
of these processes as possible, thick sections (200 µm) are required. By using silver im-pregnation (Golgi method, p. 18), individualnerve cells with a large number of processes can be demonstrated in such
thick sections. Recently, however, this 100-year-old, effec-tive method has
taken a back seat, because it is now possible to stain individual nerve cells
by filling them with a dye using rec-ording
electrodes (A). The advantage of
thistechnique is that electrical signals can be recorded from the neuron in
question at the same time. In addition to visualization by light microscopy, the intracellularly stainedor Golgi-impregnated
nerve cells can sub-sequently be examined by electron micros-copy to show the synaptic contacts of thes eneurons.
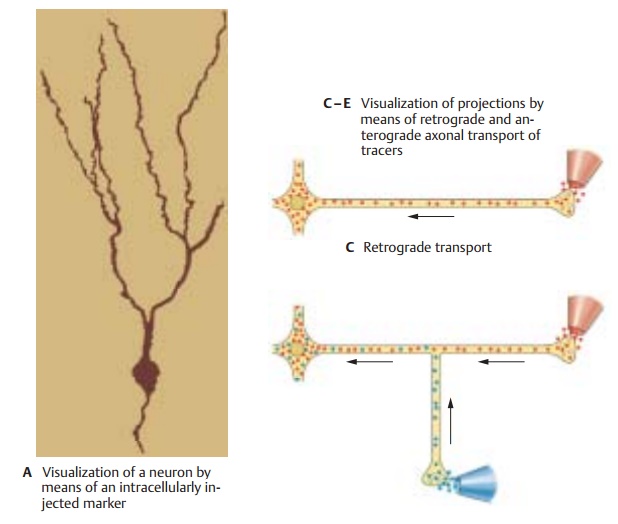
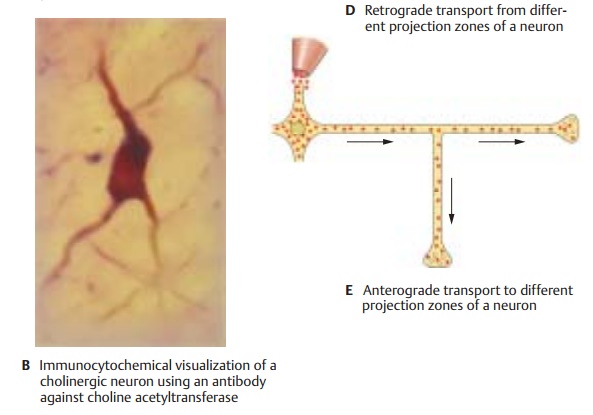
An
important characteristic of nerve cells is their specific neurotransmitter or messengersubstance
by which communication withother nerve cells is achieved. By means of immunocytochemistry and the use of anti-bodies
against the messenger substances themselves, or against neurotransmitter-synthesizing enzymes, it is possible to visual-ize
nerve cells that produce a specific trans-mitter (B). Again, these immunocytochemi-cally stained nerve cells and
their processescan subsequently be examined by electron microscopy.
The
longest processes of nerve cells, the axons (which can be up to 1 m long in
humans), cannot be traced to their target area in histological sections. In
order to demonstrate the axonal projections of neu-rons to different brain
regions, axonal trans-port (p. 28, D)
is utilized. By means of anter-ograde and retrograde axonal transport,
substances are transported from the nerve cell body to the axon terminal and
from the axon terminal back to the nerve cell body. Very long fiber connections
can be visual-ized (C – E) by means of tracers (e.g., fluorescent dyes) that are injected either into the
target area or into the region con-taining the cell bodies of the corresponding
population of neurons; the tracers are then taken up by the axon terminals or
by the cell bodies of the projection neurons, respec-tively. When using retrograde transport (C), the tracer is injected into the
assumed tar-get area. If the assumed connecting tracts exist, the tracer will
accumulate in the cell bodies. By means of retrograde transport and the use of
different fluorescent dyes (D),
different projection zones of one and
the same neuron can be demonstrated. When using anterograde transport (E),
the tracer is injected into the region of the cell bodies of projecting
neurons. Labeled axon terminals will be visible in the assumed target zone if the labeled neurons
indeed project to this area.
Tissue cultures of nerve cells are being em-ployed
to an increasing extent for studying the processes of development and
re-generation of nerve cells, and also for study-ing the effects of
pharmaceuticals.
Related Topics