Chapter: Clinical Dermatology: Skin tumours
Malignant melanoma
Malignant melanoma
Malignant
melanoma attracts a disproportionate amount of attention because it is so often
lethal. The public now knows more about its increasing incidence and dangers.
Incidence
The incidence in white people in the UK and USA is doubling every 10 years. In Scotland and northern parts of the USA the incidence is now about 10 per 100 000 per year, with females being affected more often than males.
There is a higher incidence in white people
living near the equator than in temperate zones and there the female
preponderance is lost. The high-est incidence, more than 40 per 100 000 per
year, is seen in white people living in Australia and New Zealand. The tumour
is rare before puberty and in black people, Asians and Orientals and when it
does occur in these races it is most often on the palms, soles or mucous
membranes.
Cause
Genetic.
Malignant melanomas are most common inwhite people with blond or red hair, many
freckles and a fair skin that tans poorly. Those of Celtic origin are
especially susceptible. Ten to 15% of melanomas are familial (occur in families
where two or more first-degree relatives have a melanoma). Molecular defects in
both tumour suppressor genes and oncogenes have been linked to these melanomas;
the one attracting most interest at present lies on chromosome 9p and encodes a
tumour suppressor gene designated p16, also known as CDKN2A.
Melanoma may affect sev-eral members of a single family in association with
atypical (dysplastic) naevi.
Sunlight.
Both the incidence and mortality increasewith decreasing latitude. Tumours
occur most often, but not exclusively, on exposed skin.
Pre-existing
melanocytic naevi. The risk of develop-ing a
malignant melanoma is highest in those with atypical naevi, congenital
melanocytic naevi or many banal melanocytic naevi. A pre-existing naevus is
seen histologically in about 30% of malignant melanomas.
Clinical features
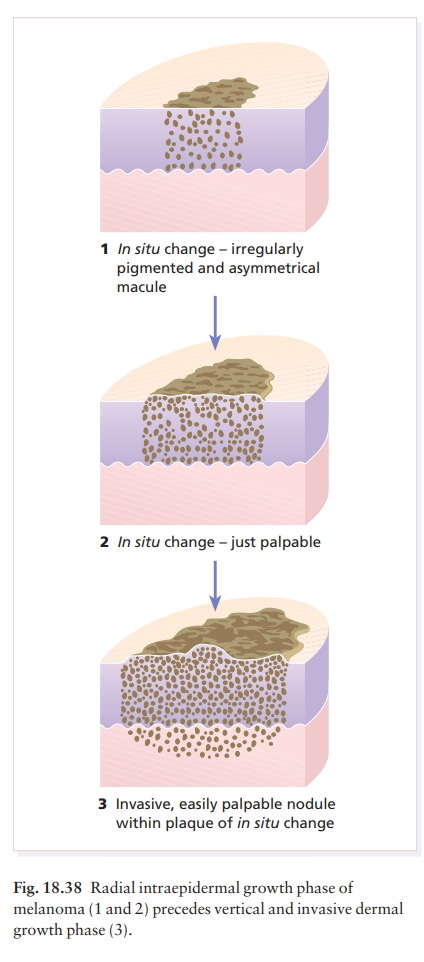
Eighty
per cent of invasive melanomas are preceded by a superficial and radial growth
phase, shown clin-ically as the expansion of an irregularly pigmented macule or
plaque (Fig. 18.38). Most are multicol-oured mixtures of black, brown, blue,
tan and pink. Their margins are irregular with reniform projec-tions and
notches. Malignant cells are at first usually confined to the epidermis and
uppermost dermis, but eventually invade more deeply and may metastasize (Fig.
18.38).
There
are four main types of malignant melanoma.
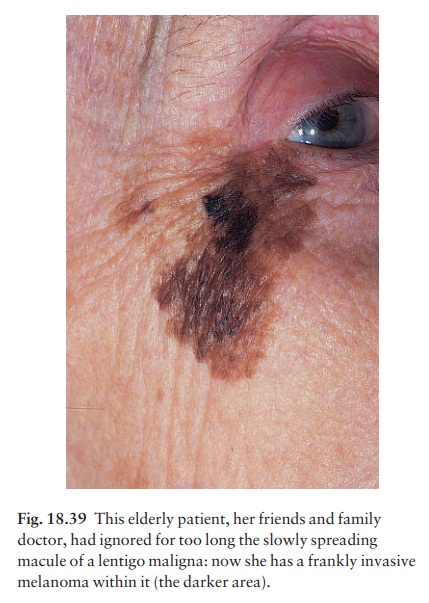
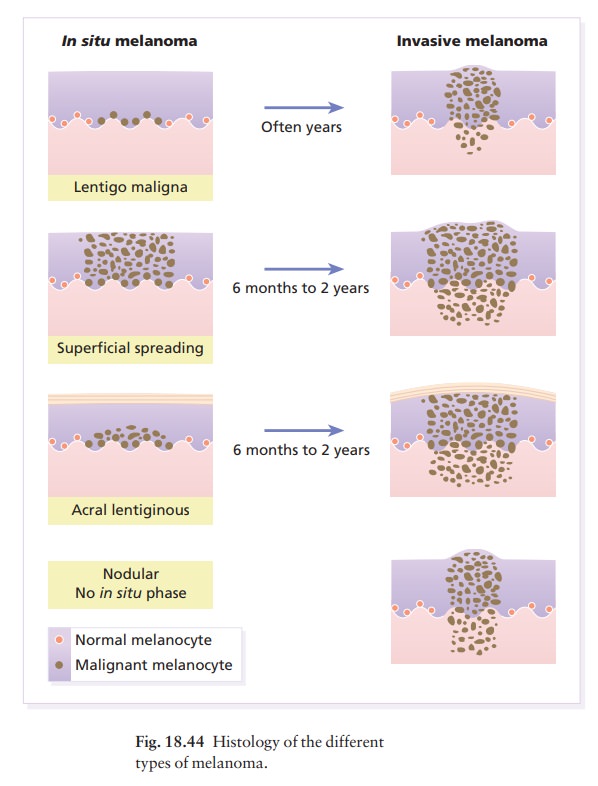
1 Lentigo
maligna melanoma occurs on the exposedskin of the
elderly. An irregularly pigmented, irregu-larly shaped macule (a lentigo
maligna) may have been enlarging slowly for many years as an in situ
melanoma before an invasive nodule (the lentigo maligna melanoma) appears (Fig.
18.39).
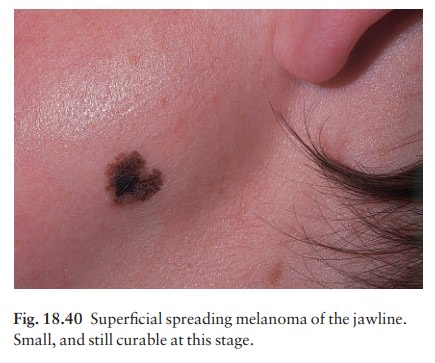
2 Superficial spreading melanomais
the mostcommon type in Caucasoids. Its radial growth phase shows varied colours
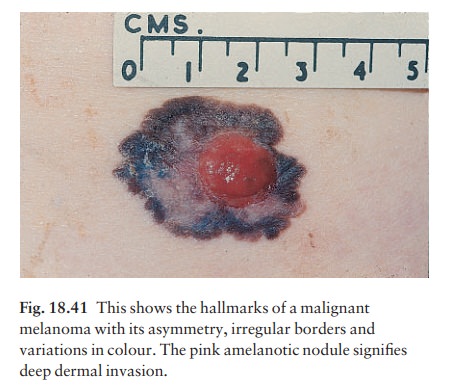
and is often palpable (Figs 18.40 and 18.41). A nodule coming up within such a
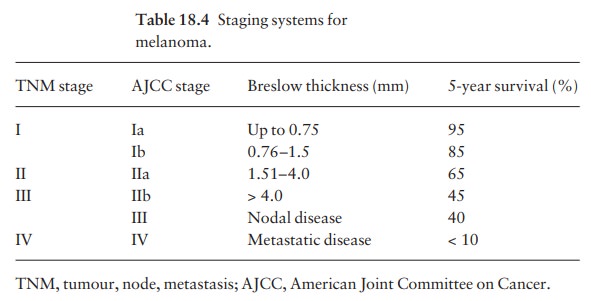
plaque signifies deep dermal invasion and a poor prognosis (Table 18.4).
3 Acral lentiginous melanomaoccurs
on the palmsand soles and, although rare in Caucasoids, is the most common type
in Chinese and Japanese people. The invasive phase is again signalled by a
nodule coming up within an irregularly pigmented macule or patch.
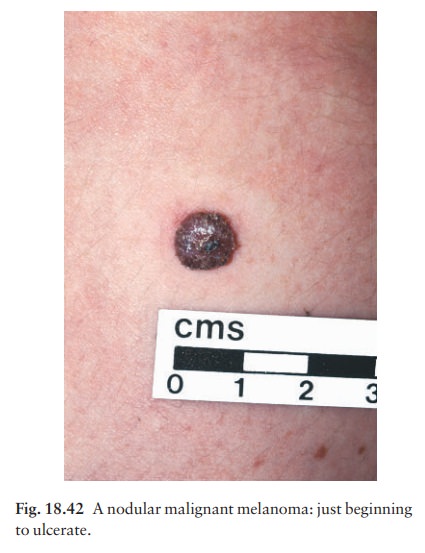
4 Nodular melanoma(Fig. 18.42) appears as a pig-mented nodule with no preceding in situ phase. It is the most rapidly growing and aggressive type.
Melanomas
can also described by their colour, site and degree of spread.
ŌĆó
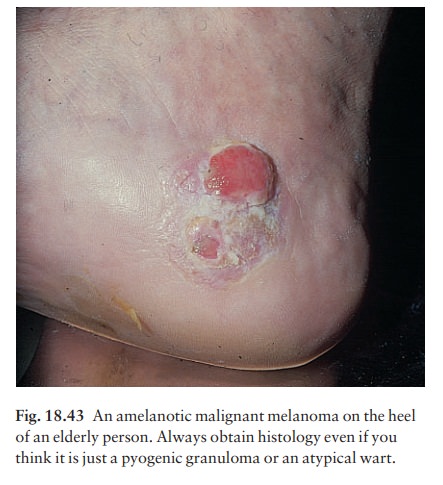
Totally amelanotic melanomas (Fig.
18.43) are rareand occur especially on the soles of the feet. Flecks of pigment
can usually be seen with a lens.
Subungual melanomas are painless areas of pig-mentation expanding under the nail and onto the nail fold.
ŌĆó
Metastatic melanoma has spread
to surroundingskin, regional lymph nodes or to other organs. At this stage it
can rarely be cured.
Staging
The most popular staging systems for melanoma are the TNM classification (Europe) and the American
Joint
Committee on Cancer (AJCC) system in the USA (Table 18.4). They provide a
useful guide to prognosis (see also Table 18.5).
Histology
ŌĆó
Lentigo maligna. Numerous atypical melanocytes,many
in groups, are seen along the basal layer extend-ing downwards in the walls of
hair follicles.
ŌĆó
Lentigo maligna melanoma. Dermal
invasion occurs,with a breach of the basement membrane region. Insitu changes
are seen in the adjacent epidermis.
ŌĆó
Superficial spreading melanoma in situ.
Largeepithelioid melanoma cells permeate the epidermis.
Superficial spreading melanoma. The dermal nodulemay be composed of epithelioid cells, spindle cells or naevus-like cells. In situ changes are seen in the adjac-ent epidermis.
ŌĆó
Acral lentiginous melanoma in situ.
Atypicalmelanocytes are seen in the base of the epidermis and permeating the
mid epidermis.
ŌĆó
Acral lentiginous melanoma. Melanoma
cells invadethe dermis. In situ changes are seen in the adjacent
epidermis.
ŌĆó Nodular melanoma. The tumour comprises epithe-lioid, spindle and naevoid cells and there is no in situ melanoma in the adjacent epidermis.
Microstaging
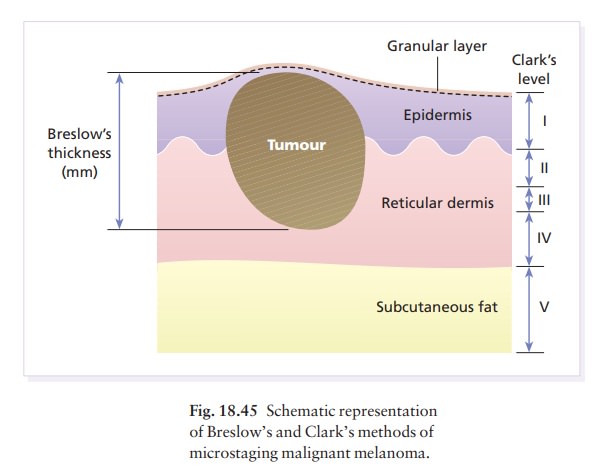
The histology (Fig. 18.44) can be used to assess prog-nosis. BreslowŌĆÖs method is to measure, with an ocular micrometer, the vertical distance from the granular cell layer to the deepest part of the tumour. ClarkŌĆÖs method is to assess the depth of penetration of the melanoma (Fig. 18.45) in relation to the different layers of the dermis. The thicker and more penetrat-ing a lesion, the worse is its prognosis .
Differential diagnosis
This
includes a melanocytic naevus, seborrhoeic ker-atosis, pigmented actinic
keratosis, pigmented basal cell carcinoma and sclerosing haemangioma;. A malignant
melanoma can also be confused with a subungual or peri-ungual haematoma (see
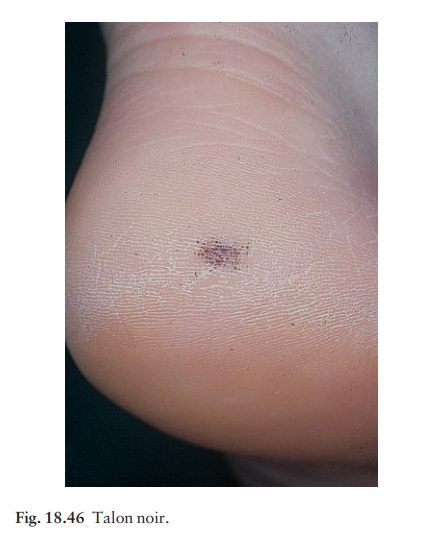
Fig. 13.21). A history of trauma helps here, as may paring. ŌĆśTalon noirŌĆÖ (Fig.
18.46) is a pigmented petechial area on the heel following minor trauma from
ill-fitting training shoes. An amelanotic melanoma is most often confused with
a pyogenic granuloma and with a squamous cell carcinoma.
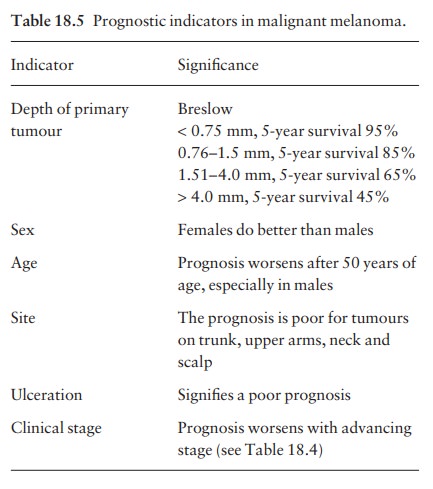
Prognosis
The
prognostic indicators, and their significance, are listed in Table 18.5. They
have been established by following up large numbers of patients who have
undergone appropriate surgical treatment .
Treatment
Surgery.
Surgical excision, with minimal delay, isrequired. An excision biopsy, with a
2-mm margin of clearance laterally, and down to the subcutaneous fat, is
recommended for all suspicious lesions. If the histology confirms the diagnosis
of malignant melanoma then wider excision, including the wound
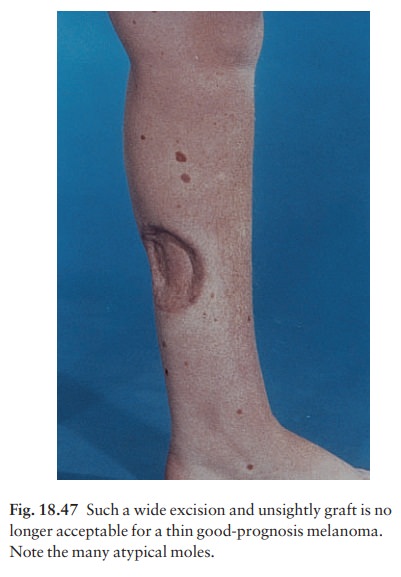
A minimum of 0.5 cm clearance for in situ melanomas and 1 cm clearance is required for all inva-sive melanomas. Nowadays many surgeons excise 1 cm of normal skin around the tumour (or wound) for every millimetre of tumour thickness, up to 3 mm (Fig. 18.47). The maximum clearance is thus 3 cm of normal skin and, depending on the site, primary closureawithout graftingais often possible.
There is no
convincing evidence that excision margins wider than 3 cm confer any greater
survival advantage. Tissue is removed down to, but not including, the deep
fascia.
Elective
regional node dissection may benefit patients with tumours of intermediate
thickness (1.5ŌĆō4.0 mm). The role of sentinel node biopsy in detecting occult
metastases is currently being investigated in patients with melanomas greater
than 1 mm thick, with the aim of carrying out elective dissection of the local
nodes in positive cases, avoiding this significant pro-cedure when the sentinel
node is not involved. The sentinel node, the first and often nearest local node
in the lymphatic drainage of the tumour, is detected by a blue dye and a
radiolabelled colloid injected intrader-mally around the tumour before excision.
The detec-tion of a positive sentinel node does correlate with prognosis but,
as yet, it remains to be shown that patients benefit from subsequent wide
dissection of the nodes in the local basin or other adjuvant treat-ment (e.g. ╬▒-interferon) after a positive sentinel node is found.
Immunological
treatments. Surgery cures most patientswith early melanoma, but its
effect on survival lessens as the disease advances. Many
ongoing trials are investigating the role of immunotherapy (e.g. with
melanoma-specific antigens) as an adjunct to surgery in patients with poor
prognostic (e.g. TNM stages II and III) melanomas. Low dose ╬▒-interferon appears to improve the disease-free survival
time and high-dose regimens may improve overall survival rates. The results of
randomized control studies of adjunct-ive treatment with various melanoma
vaccines are awaited with interest.
Chemotherapy.
Although rarely curative, chemother-apy may be palliative in 25% of patients
with stage III melanoma.
Related Topics