Chapter: Pharmaceutical Drug Analysis: Pharmaceutical Chemicals: Purity and Management
Limit Tests for Non-Metallic Impurities
LIMIT TESTS FOR NON-METALLIC IMPURITIES
Non-metallic impurities, such as boron, free halogens (I2,
Br2 and Cl2) and selenium in pharmaceutical substances
usually contribute untoward reactions, skin manifestations and are found to be
toxic to healthy tissues.
A few typical examples are described below which essentially
contains the above cited nonmetallic impurities :
1. Boron
A. Salbutamol Sulphate :
Boron
shows its presence in the above compound as a result of the use of sodium borohydride (NaBH4)
in the manufacturing process. The estimation depends upon the conversion of
boron to borate and the organic matter is subsequently destroyed by ignition
with anhydrous sodium carbonate. The quantity of boron is finally determined by
colorimetric assay.
Materials Required : Salbutamol sulphate 50 mg ;
solution of an equimolar mixture of anhydrous sodium carbonate and potassium carbonate (3% w/v in DW) 5.0 ml ;
Solution of curcumin (0.125% w/v in glacial acetic acid) 3.0 ml ; mixture of H2SO4
and glacial CH3COOH (5 ml : 5 ml) 3.0 ml ; ethanol (96%) 100 ml ;
solution of boric acid (dissolve 5 g of boric acid in a mixture of 20 ml DW and
20 ml absolute ethanol and dilute to 250 ml with absolute ethanol) : 100 ml.
Procedure : To 50 mg of substance add 5 ml
of a 3% w/v solution of an equimolar mixture of anhydrous Na2CO3 and K2CO3,
evaporate to dryness on a water-bath and dry at 120°C. Ignite the residue
rapidly until the organic matter has been destroyed, allow to cool and add 0.5
ml DW and 3 ml freshly prepared 0.125% w/v soln. of curcumin in glacial acetic
acid. Warm gently to effect solution, allow to cool and add 3 ml of a mixture
of H2SO4, with stirring, to 5 ml of glacial acetic acid.
Mix and allow to stand for 30 minutes. Add sufficient ethanol (96%) to produce
100 ml, filter and measure the absorbance of the filtrate at the maximum of 555
nm. Calculate the content of boron from a reference curve prepared from the
absorbance obtained by treating suit-able aliquots of a solution of boric acid
in the same manner.
Prescribed Limits : Not more than 50 ppm.
2. Free Halogens
A few typical examples of pharmaceutical chemicals in
which free halogens like Iodine, Bromine, Fluo-rine and Chlorine are present as
non-metallic impurities are given below.
A. Clioquinol : (Free Iodine)
Materials Required : Clioquinol 1.0 g ; potassium
iodide 1.0 g ; H2SO4
(1 M) 1.0 ml ; chloroform 2.0 ml
; sodium thiosulphate (0.005 M) 0.1 ml.
Procedure : Shake 1.0 g with a solution of
1 g potassium iodide in 20 ml DW for 30 seconds, allow to stand for 5 minutes and filter. To 10 ml of the filtrate add 1 ml
1 M H2SO4 and 2 ml chloroform and shake.
Prescribed Limits : Any colour in the chloroform
layer is discharged on the addition of 0.1 ml of 0.005 M sodium thiosulphate.
B. Diethylpropion
Hydrochloride : (Free
Bromine)
Test : Place 0.05 ml of a 10% w/v
solution on starch-iodide paper.
Prescribed Limit : No colour is produced.
C. Doxycycline Hydrochloride :
(Free
Fluorine)
Materials Required : Doxycyline Hydrochloride :
0.30 g ; oxygen-combustion flask ; 1 L capacity;
Nessler cylinder 100 ml ; zirconyl alizarin solution* :
5.0 ml ; fluoride standard solution (10 ppm F) (dilute 5.0 ml of a 0.0442 % w/v
soln. of sodium fluoride, previously dried at 300°C for 12 hours, to 100 ml
with DW) : 3.0 ml.
Procedure : Burn 0.30 g, in three equal
portions, by the method for oxygen-flask combustion (BP), using a 1 Litre flask and a separate 20 ml portion of DW as the
absorbing liquid for each combustion, shaking the flask vigorously for about 15
minutes and transferring to the same 100 ml Nessler cylinder. Add 5 ml of acid
zirconyl alizarin solution to the combined liquids, adjust the volume to 100 ml
with DW and allow to stand for 1 hour.
Prescribed Limit : The colour of the resulting
solution is greater than that obtained by repeating the operation with no substance enclosed in the successive portions of
filter paper burnt in the method for oxygen flask combustion, but adding 3.0 ml
of fluoride standard solution (10 ppm F) to the combined absorption liquids
before adding the acid zirconyl alizarin solution.
D. Chloroform : (Free Chlorine)
Materials Required : Chloroform 10.0 ml ; cadmium
iodide solution (5.0% w/v in DW) 1.0 ml ; starch mucilage 0.1 ml.
Procedure : Shake 10 ml of chloroform with
20 ml of freshly boiled and cooled DW for 3 minutes and allow to separate. To the aqueous layer add 1 ml cadmium iodide
soln. and 0.1 ml of 10 ml of starch mucilage.
Prescribed Limit : No blue colour is produced.
E. Tetrachloroethylene (Free Chlorine)
Perform the limit test as stated under chloroform. No
blue colour is produced.
3. Selenium
A. Selenium Sulphide
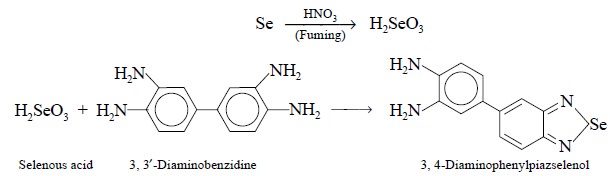
Theory : Selenium is very toxic and its
contamination is usually controlled by an absorptiometric method after destruction of the organic
compound with fuming nitric acid. The latter converts selenium (Se) as selenous
acid (H2SeO3), which on subsequent treatment with 3,3′-diaminobenzidine under
controlled experimental pa-rameters, results into the formation of a highly
coloured compound known as 3,4-diaminophenylpiazselenol. The latter is
consequently extracted with toluene after making the aqueous solution alkaline,
and the colour compared with a standard prepared likewise from a known amount
of selenium. The various reactions involved may be expressed as follows :
Materials Required : Selenium sulphide : 10.0 g ;
formic acid (2.5 M) : 2.0 ml ; 3,3′-diaminobenzedine tetrahydrochloride
solution (0.5% w/v in DW) : 2.0 ml ; ammonia (5 M) : 20 ml ; selenium standard
solution (1 ppm Se) (Dilute 2.5 ml of a 0.00654% w/v solution of selenous acid
to 100 ml with DW) : 5.0 ml.
Procedure : To 10 g of selenium sulphide
add 100 ml DW, mix well, allow to stand for 1 hour with frequent shaking and filter. To 10 ml of the filtrate, add 2 ml of
2.5 M formic acid, dilute to 50 ml with DW, adjust the pH to 2.0 to 3.0 with
2.5 M formic acid, add 2.0 ml of a 3,3′-diaminobenzidine
tetrahydrochloride in DW, allow to stand for 45 minutes and adjust the pH to
6.0 to 7.0 with 5 M ammonia. Shake the solution for 1 minute with 10 ml of
toluene and allow to separate. Measure the absorbance at 420 nm.
Prescribed Limit : The measured absorbance at 420
nm is not greater than that of a solution prepared by treating 5 ml of selenium standard solution (1 ppm Se) in the
same manner (5 ppm, calculated as Se).
Related Topics