Chapter: Pharmaceutical Drug Analysis: Pharmaceutical Chemicals: Purity and Management
Pharmaceutical Chemicals: Purity
PURITY
The standardization of ŌĆśpharmaceutical chemicalsŌĆÖ and the dosage forms prepared therefrom
plays a vital role so that the patient gets the ŌĆśdrugŌĆÖ within the permissible
limits of potency and tolerance.
The standards for pharmaceutical chemicals and their
respective dosage forms, as laid down in, various
Official Compendia fulfil broadly the following three cardinal objectives, namely :
(a) Broad-based
highest attainable standard,
(b) Biological
response versus chemical purity, and
(c) Offical
standards versus manufacturing
standards.
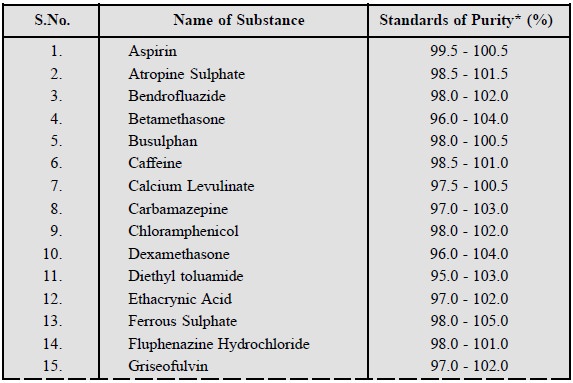
1. BROAD-BASED HIGHEST ATTAINABLE STANDARD
Keeping in view the various methods of manufacture of a
pharmaceutical substance vis-a-vis
its standards of purity, types of impurity and changing pattern of stability, a
broad-based highest attainable standard is always fixed. A few typical examples
are stated below :

2. BIOLOGICAL RESPONSE VS. CHEMICAL PURITY
Though chemical purity is the topmost priority, yet the
biological response of a pharmaceutical substance holds an equal importance. A
wide variation of active ingredients ranging between 90% in one sample and 110%
(┬▒ 10 per cent limit) in another sample could invariably be observed.
Therefore, it has become absolutely essential to lay down definite standards so
as to ensure that :
┬Ę
Different laboratories may produce reasonably reproducible
products.
┬Ę
Difference in active ingredients in various lots may be
minimised.
┬Ę
Retention of acceptable level of potency.
┬Ę
Freedom of toxicity during storage before use.
Examples :
(i) Substances
to be stored in well-closed, light-resistant containers e.g., isoniazid, nalidixic acid, nandrolone phenylpropionate,
nitrofurazone.
(ii) Substances
to be stored under nitrogen in tightly closed, light-resistant containers at a
temperature between 2┬░ and 10┬░C, e.g.,
nandrolone decanoate, nystatin, methylergometrine maleate, human normal
immunoglobulin.
(iii)
Substances to be stored in tightly-closed, light-resistant containers in a cool
place, e.g., nitrofurantoin,
pancreatin, oxyphenonium bromide.
(iv) Substances
to be stored in tightly-closed, light-resistant containers in a cool place; for
parenteral administration, the container should be sterile and sealed so as to
exclude micro-organisms. e.g.,
kanamycin sulphate, novobiocin sodium, benzylpenicillin, lincomycin
hydrochloride, chloramphenicol.
(v) Substances
to be stored in well-closed containers, at a temperature not exceeding 30┬░C, e.g., procaine penicillin, pepsin,
menthol, erythromycin.
3. OFFICIAL STANDARDS VIS-A-VIS MANUFACTURING STANDARDS
The Offical
Standards, as stipulated in the pharmacopoeias of various countries, e.g., IP BP, Eur. P., Int. P., USSRP, JP
etc., of a pharmaceutical substance take cognizance of the purity, nature,
methods and haz-ards of manufacture, precautions of storage and ultimately the
conditions under which the product is to be used.
It is a well-known fact that a pharmaceutical substance
can be prepared by adopting different routes of synthesis based upon the
dynamic ongoing research in the field of organic-reaction-mechanisms.
Relentless efforts are exerted vigorously by reputed research laboratories
across the world to look for shorter routes of synthesis bearing in mind the
cost-effectiveness of the final product. For instance : diclofenac sodium (an
NSAID) can be manufactured by two methods, one using a bromo compound as a
starting material while the other is based on a non-bromo compound.
Nevertheless, the latter product is more in demand because it is completely
devoid of bromine residues in the final product.
During the process of manufacture an unavoidable criterion
is the loss of active ingredients. Therefore, all Official Standards for pharmaceutical chemicals and dosage forms
should accomodate such losses caused due to loss in manufacture, unavoidable
decomposition and storage under normal conditions for a stipulated period.
It has become an usual
practice to include a ŌĆśdefinite overageŌĆÖ in certain dosage forms so as to
compensate the noticeable losses caused either due to manufacturing or storage
(anticipated decomposition), in order that the finished product may comply with
the prescribed offcial standards after the stipulated duration of storage.
Official standards with regard to dosage form and packs,
preservation and prevention from contamination in a variety of pharmaceutical
products, such as eye-drops, multidose injections and antiseptic creams
(external application) that may be prone to spoilage with prolonged repetitive
usage should be well defined. The official standards, in general, legislate and
control the presence of toxic impurities by prescribed ŌĆślimit testsŌĆÖ and also by more sophisticated analytical techniques
using thin-layer chromatography (TLC), high performance thin-layer
chromatography (HPTLC), gas-liquid chromatography (GLC) and high-performance
liquid chromatography (HPLC).
Related Topics