Chapter: Pharmaceutical Drug Analysis: Pharmaceutical Chemicals: Purity and Management
Physical Constants - Pharmaceutical Chemicals: Management
PHYSICAL CONSTANTS
A wide range of physical constants, for instance :
melting point, boiling point, specific gravity, viscosity, refractive index,
solubility, polymorphic forms vis-a-vis
particle size, in addition to characteristic absorption features and optical
rotation play a vital role in characterization of pharmaceutical chemicals and
drug substances. These physical constants will be discussed briefly with
typical examples as under :
1. Melting Point
It is an important criterion
to know the purity of a substance ; however, it has a few limitations. The
accuracy and precision of melting point is dependent on a number of factors
such asŌĆöcapillary size, sample size, initial temperature of heating-block and
the rate of rise of temperature per unit time (minutes). Keeping in view the
different manufacturing processes available for a particular drug the melting
point has a definite range usually known as the melting range.
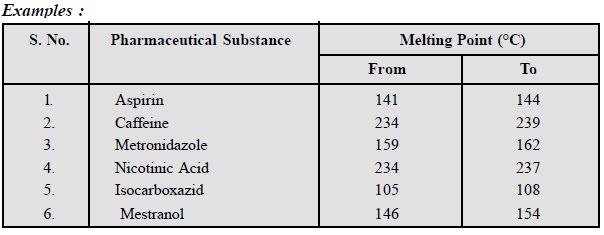
Thus the melting range takes care of the variance in manufacture
together with the storage variance over a stipulated period of time.
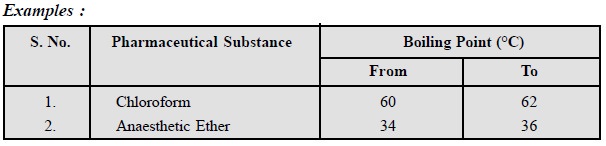
2. Boiling Point
It is also an important
parameter that establishes the purity of a substance. Depending on the various
routes of synthesis available for a substance a boiling point range is usually
given in different official compendia.
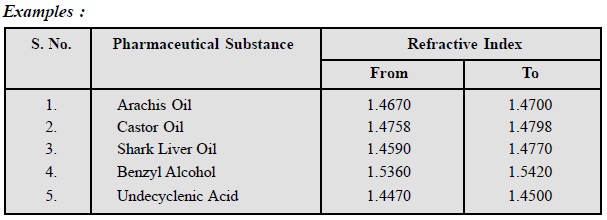
3. Refractive Index
It is invariably used as a
standard for liquids belonging to the category of fixed oils and synthetic
chemicals.
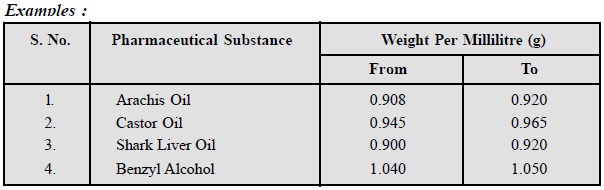
4. Weight Per Millilitre
Weight per millilitre is
prevalent in the Pharmacopoeia of India for the control of liquid substances,
whereas Relative Density (20┬░/20┬░) or Specific Gravity is mostly employed in
the European Pharmacopoeia.
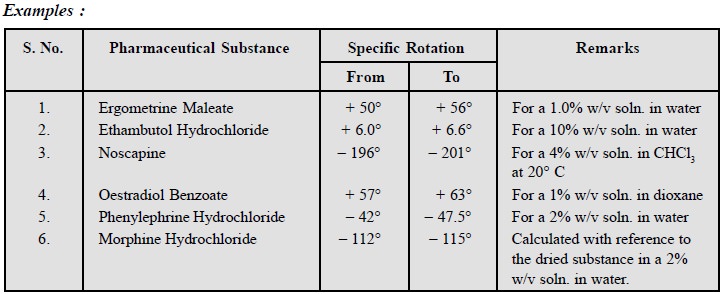
5. Specific Optical Rotation
As pharmacological activity is
intimately related to molecular configuration, hence determination of specific
rotation of pharmaceutical substances offer a vital means of ensuring their
optical purity.
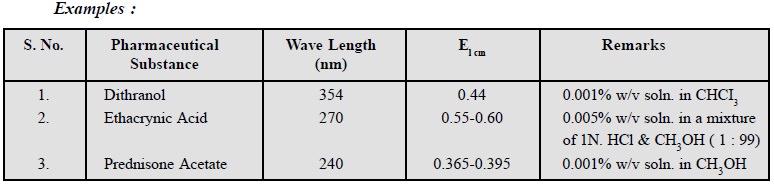
6. Light Absorption
The measurement of light
absorption both in the visible and ultraviolet range is employed as an
authentic means of identification of offcial pharmaceutical substances.
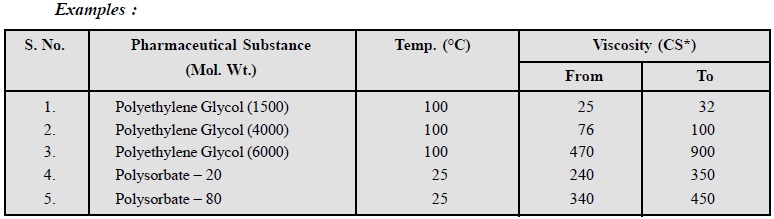
7. Viscosity
Viscosity measurements are employed as a method of
identifing different grades of liquids.
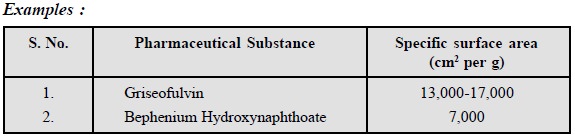
8. Specific Surface Area
The surface area of powders is determined by
subsieve-sizer which is designed for measurement of average particle sizes in
the range of 0.2 to 50 microns. The relationship between average particle
diameter and specific surface area (SSA) is given by the following expression :
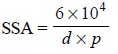
where, SSA = Specific surface area in cm2 per
g of material
d = Average diameter in microns
p = True density of material
from which the powder was made in g per cm3
9. Swelling Power
The swelling power of some pharmaceutical products are
well defined.
Examples :
(i) Isphagula Husk : When 1 g, agitated
gently and occasionally for four hours in a 25 ml stoppered measuring cylinder
filled upto the 20 ml mark with water and allowed to stand for 1 hour, it
occupies a volume of not less than 20 ml and sets to a jelly.
(ii) Heavy Kaolin : When 2 g is titurated
with 2 ml of water the mixture does not flow.
10. Infrared Absorption
Measurement and subsequent comparison of the infrared
spectrum (between 4000-667 cmŌĆō1) of compounds with that of an
authentic sample has recently become a versatile method for the identification
of drugs having widely varying characteristics.
Examples : Infrared spectroscopy is
employed to compare samples of chloramphenicol palmitate (biologically active
form) recovered from chloramphenicol palmitate mixture vis-a-vis an artificially prepared mixture of authentic sample
consisting 10 per cent of the ŌĆśinactive polymorphŌĆÖ.
Infrared spectra of known and newly reported compounds
are provided in the British Pharmacopoeia (1998) and also in ŌĆśSadtler Standard SpectraŌĆÖ published by
Sadtler Research Laboratories, Philadelphia
(USA) is available to check the authenticity of pure drug
samples.
11. Miscellaneous Characteristics
A large number of miscellaneous characteristics are
usually included in many official
compendia to ascertain the purity, authenticity and identification of
drugsŌĆöincluding : sulphated ash, loss on drying, clarity and colour of
solution, presence of heavy metals and specific tests.
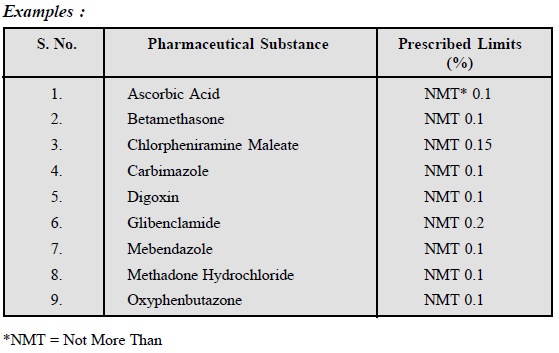
11.1. Sulphated Ash
Specifically for the synthetic organic compounds, the
Pharmacopoeia prescribes values for sulphated ash. The sulphated ash is
determined by a double ignition with concentrated sulphuric acid. Metals thus
remain as sulphides that are usually stable to heat. The method is one of some
precision, and provides results which are rather more reproducible than those
obtained by simple ignition.
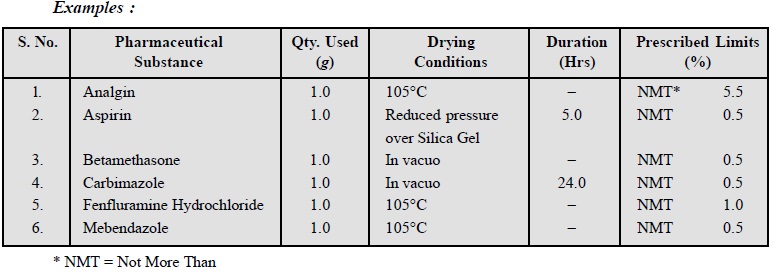
11.2. Loss on Drying
Loss on drying reflects the net weight of a
pharmaceutical substance being dried at a specified tempera-ture either at an
atmospheric or under reduced pressure for a stipulated duration with a specific
quantity of the substance.
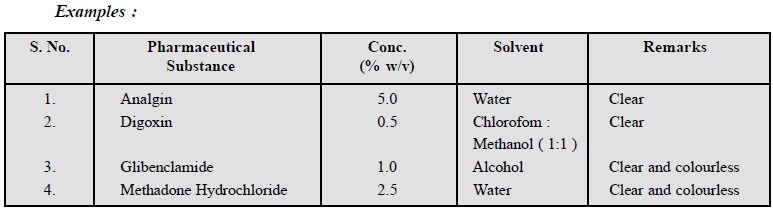
11.3. Clarity and Colour of Solution
When a pharmaceutical substance is made to dissolve at a
known concentration in a specified solvent it gives rise to a clear solution
that may be either clear or possess a definite colouration.
11.4. Heavy Metals
Various tests are prescribed in the offcial compendia to
control heavy metal e.g., Ag+,
Hg2+, Pb2+, Bi2+, Cu2+, As3+,
, Sb3+ and Sn4+ contamination in organic pharmaceutical
substances. Hence, a stringent limit is recommended for the presence of heavy
metals in medicinal compounds.
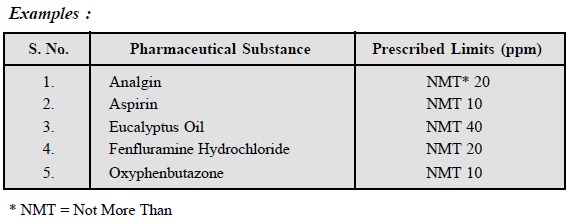
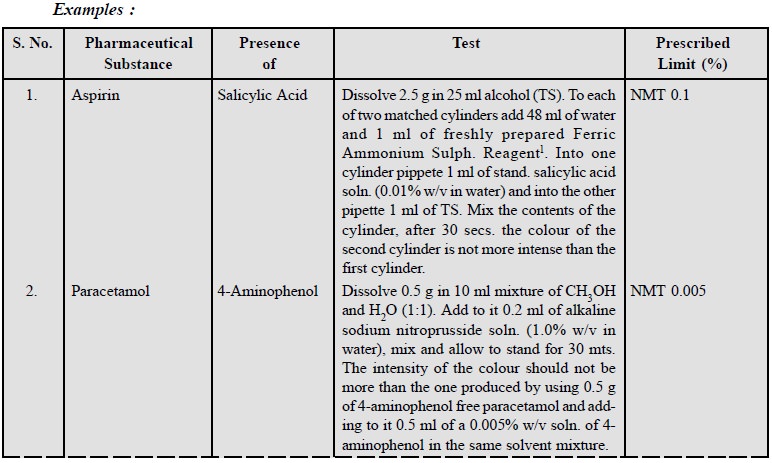
11.5. Specific Tests
In fact, certain known impurities are present in a number
of pharmaceutical substances. The presence of such impurities may be carried
out by performing prescribed specific tests in various official compendia in order to ascertain their presence within the
stipulated limits.
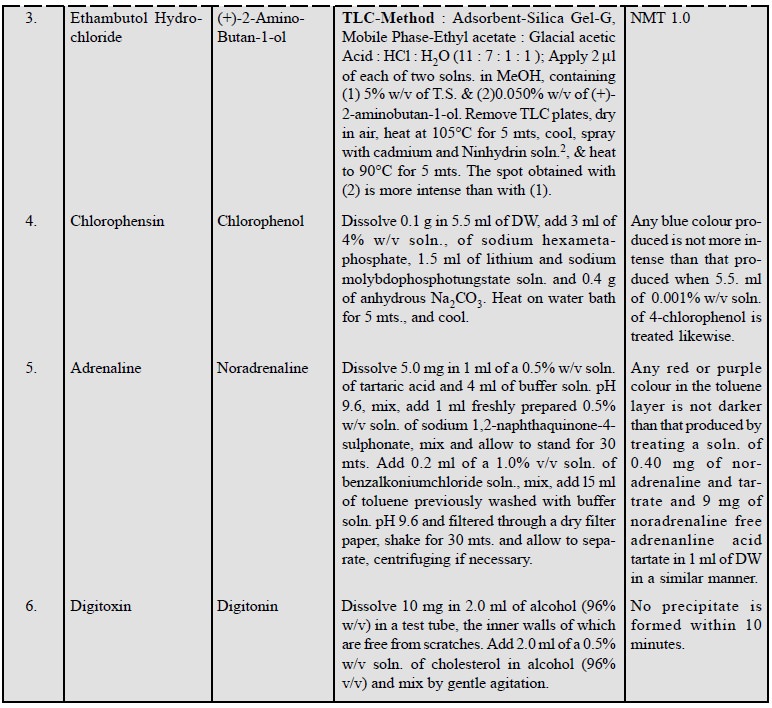
Reagents :
┬Ę
Dilute 1 ml N. HCl and 2.0 ml ferric ammonium sulphate
soln. (10% w/v in H2O) with suffcient water to produce 100 ml.
┬Ę
Dissolve 50 mg cadmium acetate in a mixture of 5 ml DW
and 1 ml glacial acetic acid and dilute with ethyl methyl ketone to 50 ml.
Immediately before use add and dissolve suffcient Ninhydrin to produce a soln.
containing 0.2% w/v.
┬Ę
Dissolve 10.0 g sodium tungstate and 2.5 g sodium
molybdate in 80.0 ml DW in a 250 ml flask; add 5.0 ml phosphoric acid (85-90%
w/w) and 10.0 ml HCl (= 11.5 N), connect to a reflux condenser and heat for 10
Hrs. Cool, add 15.0 g lithium sulphate, 5.0 ml DW and 1 drop of bromine and
allow to stand for 2 Hrs. Remove the excess bromine by boiling the mixture for
15 mts. without the condenser. Cool, filter and dilute with DW to produce 100
ml.
Caution :
(i) The prepared soln. should be stored below 4┬░C, and
(ii) The soln.
should be used within 4 months after preparation till it retains its original
golden yellow colour. It must be rejected if it has a trace of green colour.
Related Topics