Chapter: Pharmaceutical Drug Analysis: Pharmaceutical Chemicals: Purity and Management
Limit Test for Arsenic
Limit Test for Arsenic
Theory : The official process is a
development of the Gutzeit Test wherein
all arsenic present is duly converted
into arsine gas (AsH3) by subjecting it to reduction with zinc and
hydrochloric acid. Further, it depends upon the fact that when arsine comes
into contact with dry paper permeated with mercuric (Hg2+) chloride
it produces a yellow strain, the intensity of which is directly proportional to
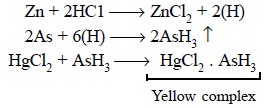
the quantity of arsenic present. The various chemical reactions involved may be
expressed by the following equations :
The details of experimental procedure described in the
Pharmacopoeia are actually based upon a paper by Hill and Collins**, but have
been adequately modified from time to time in accordance with the accumu-lated
and acquired experience. Explicitly, the expressions provided in the
Pharmacopoeia for limits of arsenic exclusively refer to parts per million,
calculated as As.
Materials Required : Arsenic limit test apparatus;
HgCl2—paper : smooth white filter paper (having thickness in mm of 400 paper = weight in g per Sq. M.), soaked in
a saturated solution of HgCl2, pressed to get rid of excess of soln.
and dried at about 60°C in the dark ; lead acetate solution 10.0% w/v soln. of
PbAc2 in CO2– free water ; KI (AsT), 1.0 g ; Zn (AsT) :
l0.0 g ; Dilute Arsenic solution (AST); Standard stains, Test Solutions—are
prepared according to the Indian Pharmacopoeia 1996.
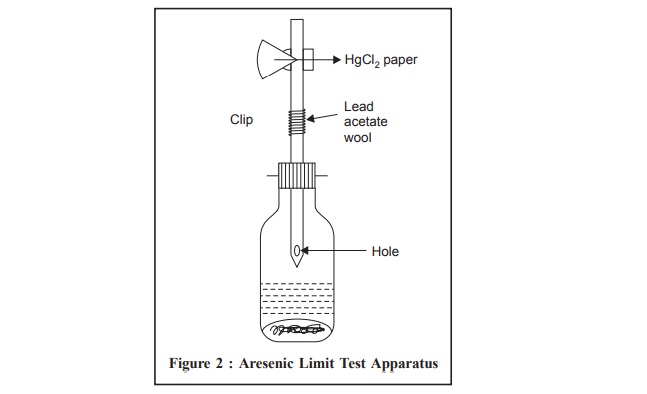
Arsenic Limit Test Apparatus (Figure 2)
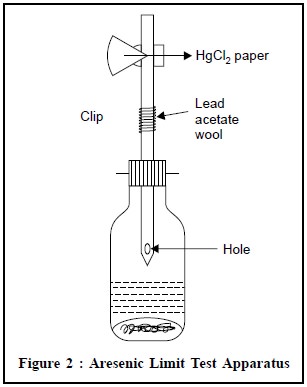
A wide-mouthed glass bottle capable of holding about 120
ml is fitted with a rubber bung through which passes a glass tube. The latter,
made from ordinary glass tubing, has a total length of 200 mm and an internal
diameter of exactly 6.5 mm (external diameter about 8 mm). It is drawn out at
one end to a diameter of about 1 mm and a hole not less than 2 mm in diameter
is blown in the side of the tube, near the constricted part. When the bung is
inserted in the bottle containing 70 ml of liquid, the constricted end of the
tube is kept above the surface of the liquid, and the hole in the side is below
the bottom of the bung. The upper end of the tube is cut off square, and is
either slightly rounded or ground smooth.
The rubber bungs (about 25 mm Ă— 25 mm), each with a hole bored centrally and through exactly 6.5 mm in diameter,are fitted with a rubber band or spring clip for holding them tightly in place.
Procedure : The glass tube is lightly
packed with cotton wool, previously moistened with lead acetate solution and dried, so that the upper
surface of the cotton wool is not less than 25 mm below the top of the tube.
The upper end of the tube is then inserted into the narrow end of one of the
pair of rubber bungs, to a depth of l0 mm (the tube must have a rounded-off
end). A piece of mercuric chloride paper is placed flat on the top of the bung
and the other bung placed over it and secured by means of the spring clip in
such a manner that the holes of the two bungs meet to form a true tube 6.5 mm
diameter interrupted by a diaphragm of mercuric chloride paper.
The test solution prepared as specified, is placed in the
wide-mouthed bottle, 1 g of KI (AsT) and 10 g of Zn (AsT) are added, and the
prepared glass tube is placed quickly in position. The reaction is allowed to
proceed for 40 minutes. The yellow stain that is produced on the HgCl2
paper if As is present is compared by daylight with the standard stains
obtained by performing in an identical manner with known quantities of dilute
arsenic solution (AsT). The comparison of the stains is made immediately at the
completion of the test.
By matching the intensity and depth of colour with
standard stains, the proportion of arsenic in the substance may be estimated.
Thus, a stain equivalent to the 1 ml standard stain obtained by performing on
l0 g of a substance implies that the proportion of As is 1 part per million.
Cautions :
(i) HgCl2 paper should be protected from
sunlight during the test to avoid lighter or no
stain.
(ii) The
standard and test stains must be compared immediately as they fade out on
retaining.
(iii) The
reaction may be expedited by the application of heat and 40°C is considered to
be the most ideal temperature.
(iv) The tube
should be washed with HCl (AsT), rinsed with DW, and dried between successive
tests.
Special Techniques : The special techniques are
usually applicable to a host of pharmaceutical sub-stances before the normal
test can be performed. A few typical examples would be discussed briefly here,
namely :
(i) Free Acids : They are first converted
to their respective sodium salts with Na2CO3 and As3+
oxi-dised to As5+ by evaporating the solution with Br2.
The residue is ignited carefully until carbonised to destroy organic matter,
while As is kept as non-volatile sodium arsenate. The resulting residue is
dissolved in brominated HCl and the test carried out in the normal manner.
Examples : Aspirin, Saccharin, Sodium
Salicylate, Sodium Aminosalicylate.
(ii) Substances Reacting Vigorously with HCl :
The As is readily converted to AsCl3 which being volatile in nature
is also carried off along with relatively large volumes of CO2
(generated by the substance and HCl).
Examples : Magnesium
Carbonate, Light Magnesium Oxide, Calcium Hydroxide, Chalk, KOH, NaOH.
(iii) Insoluble Substances : These
substances, as those that do not interfere with the solution of As and its
subsequent reduction to AsH3 (arsine). Such substances are suspended
in water along with stannated-HCl, and the normal test is performed.
Examples : Magnesium Trisilicate,
Bentonite, Barium Sulphate, Light and Heavy Kaolin.
(iv) Metals Interfering with Normal Reaction
(a) Iron : It gets deposited on the surface
of Zn thereby depressing the intensity of reaction between Zn and HCl to
produce H2.
Remedy : The sample is dissolved in H2O
and stannated HCl to allow conversion of all As to As3+ and finally as AsCl3. The latter being
volatile in nature can be separated by distillation from remaining metallic
salts and the distillate examined in the normal manner.
Example : Ferrous
Sulphate.
(b) Antimony : Sb-compounds are also
reduced simultaneously by Zn/HCl to yeild SbH3 (stilbine) that
reacts with HgCl2 paper to give a stain. Therefore, the sample is
first distilled with HCl to yield a distillate containing all the As as AsC3
(volatile), but yields only a fraction of Sb as SbCl3
(non-volatile). A repeated distillation obviously gets rid of even the last
traces of Sb.
Examples : Antimony
Potassiun Tartrate, Antimony Sodium Tartrate.
A few typical examples are cited below from the official compendium.
Limit Test for Iron
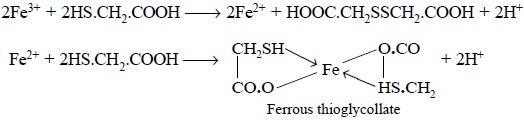
Theory : The limit test for Iron is
based on the reaction between iron and thioglycollic acid in a medium buffered with ammonium citrate to give
a purple colour, which is subsequently compared with the standard colour
obtained with a known amount of iron (0.04 mg of Fe). Ferrous thioglycollate is
a co-ordination compound that
attributes the purple colour ; besides thioglycollic acid converts the entire
Fe3+ into Fe2+. The reactions involved may be expressed
as follows :
Materials Required
Nessler cylinder : 1 pair ; Ferric ammonium sulphate :
1.726 g ; Sulphuric acid (0. 1 N) : 10.0 ml ; Iron-free citric acid (20% w/v) :
2.0 ml ; Thioglycollic acid : 0.1 ml; Iron-free ammonia solution : 20 ml.
Standard Iron Solution : Weigh accurately 0.1726 g of
ferric ammonium sulphate and dissolve in
10 ml of 0.1 N sulphuric acid and suffcient water to produce 1 Litre. Each
ml of this solution contains 0.02 mg of Fe.
Standard Colour : Dilute 2.0 ml of standard iron solution with 40 ml DW
in a Nessler cylinder. Add 2 ml of a
20% w/v solution of iron-free citric acid and 0.1 ml of thioglycollic acid,
mix, make alkaline with iron-free ammonia solution, dilute to 50 ml with DW and
allow to stand for 5 minutes.
Procedure : Dissolve the specified
quantity of the substance being examined in 40 ml DW, and trans-fer to a
Nessler cylinder. Add to it 2 ml iron-free citric acid solution and 0.1 ml
thioglycollic acid, mix, make alkaline with iron-free ammonia solution, dilute
to 50 ml with DW and allow to stand for 5 minutes. Any colour produced is not
more intense than the standard colour.
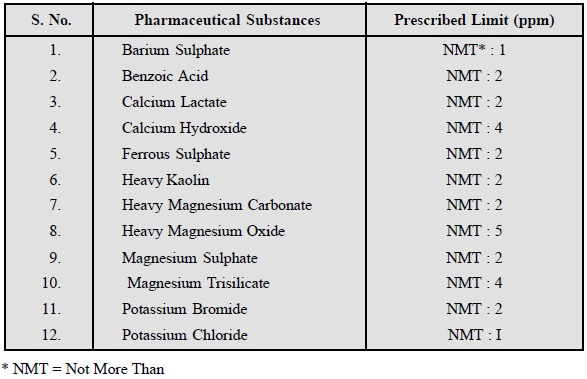
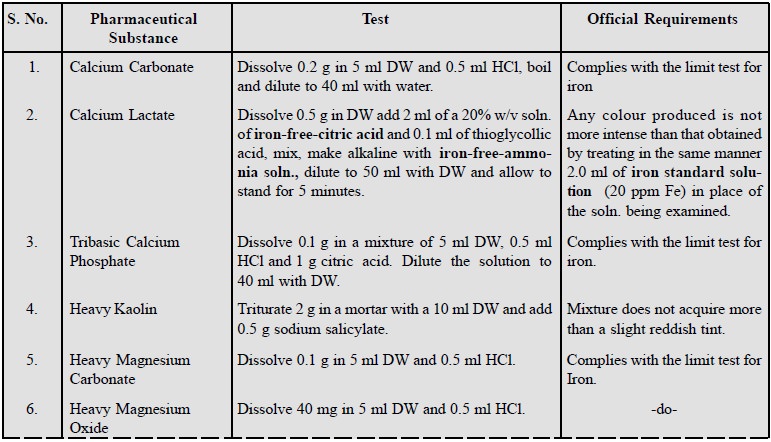
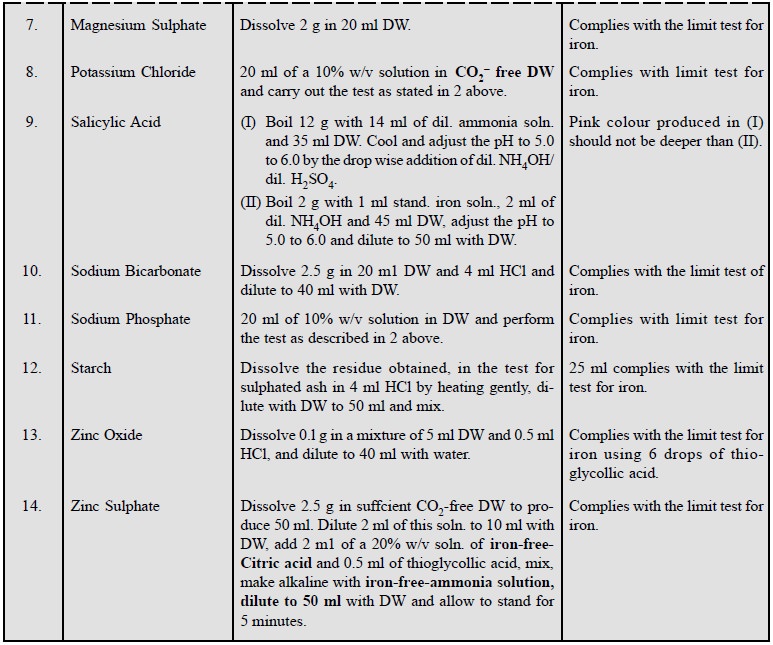
Some examples of this test for pharmaceutical substances
are listed below :
Related Topics