Chapter: Pharmaceutical Drug Analysis: Pharmaceutical Chemicals: Purity and Management
Limit Test’s for Acid Radical Impurities
LIMIT TEST’S FOR ACID RADICAL IMPURITIES
Acid radical impurities constitute a serious but
unavoidable source of impurities in a large number of pharmaceutical chemicals.
However, the two most commonly found acid radical impurities are chloride (Cl–
) and sulphate (SO42–) that evidently arise from the
inevitable use of raw tap-water in various manufacturing operations. As these
two acid radical impurities are found in abundance due to contamination, the
Pharmaco-poeia categorically stipulates limit tests for them which after due
minor modifications are applicable to a number of pharmaceutical substances.
In addition to the above two commonly found impurities,
there are a number of other acid radical impurities which exist in
pharmaceutical substances, namely : arsenate, carbonate, cyanide, nitrate,
oxalate, phosphate and silicate.
All these acid radical impurities shall be discussed
briefly as under :
1. Limit Test for Chlorides
The limit test for chlorides is based on its
precipitation with silver nitrate in the presence of dilute HNO3,
and comparing the opalescence produced due to the formation of AgCl with a
standard opalescence achieved with a known quantity of Cl– ions.
The equation may be expressed
as :
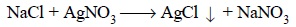
Materials Required : Nessler cylinder 1 pair ;
dilute nitric acid (10% w/w of HNO3) 10.0 ml ; silver nitrate solution (5.0% w/v in DW) 1.0
ml.
Standard Opalescence : Place 1.0 ml of a 0.05845% w/v
solution of NaCI in 10 ml of dilute HNO3 in a Nessler cylinder.
Dilute to 50 ml with DW and add 1 ml of AgNO3 solution. Stir
immediately with a glass rod and allow to stand for 5 minutes.
Procedure : Dissolve the specified quantity
for the substance in DW, or prepare a solution as directed in the text and transfer to a Nessler cylinder. Add 10 ml of
dilute nitric acid, except when it is used in the preparation of the solution,
dilute to 50 ml with DW, and add 1 ml of AgNO3 solution. Stir
immediately with a glass rod and allow to stand for 5 minutes. The opalescence
produced is not greater than the standard opalescence, when viewed
transversely.
A few typical examples of this
test representing a wide spectrum of pharmaceutical substances are enumerated
below :
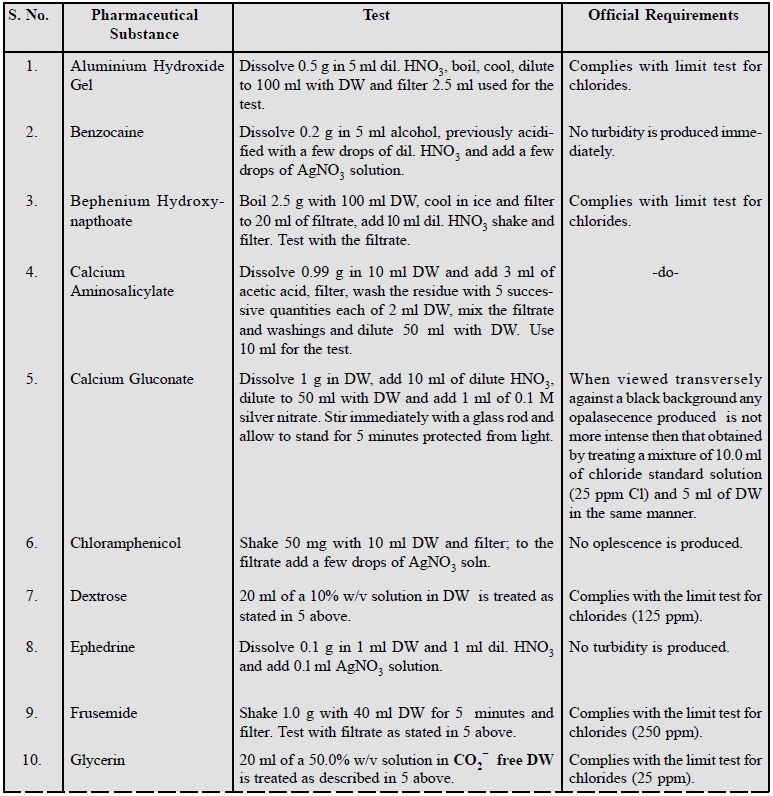
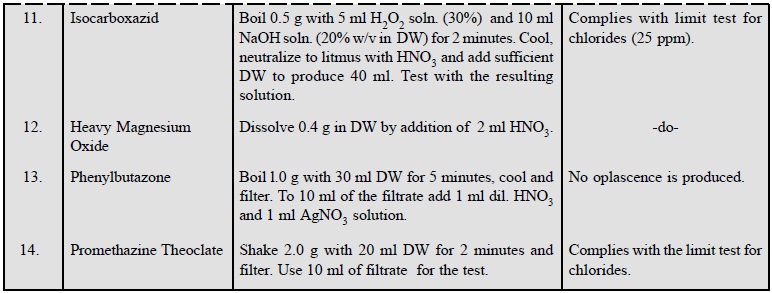
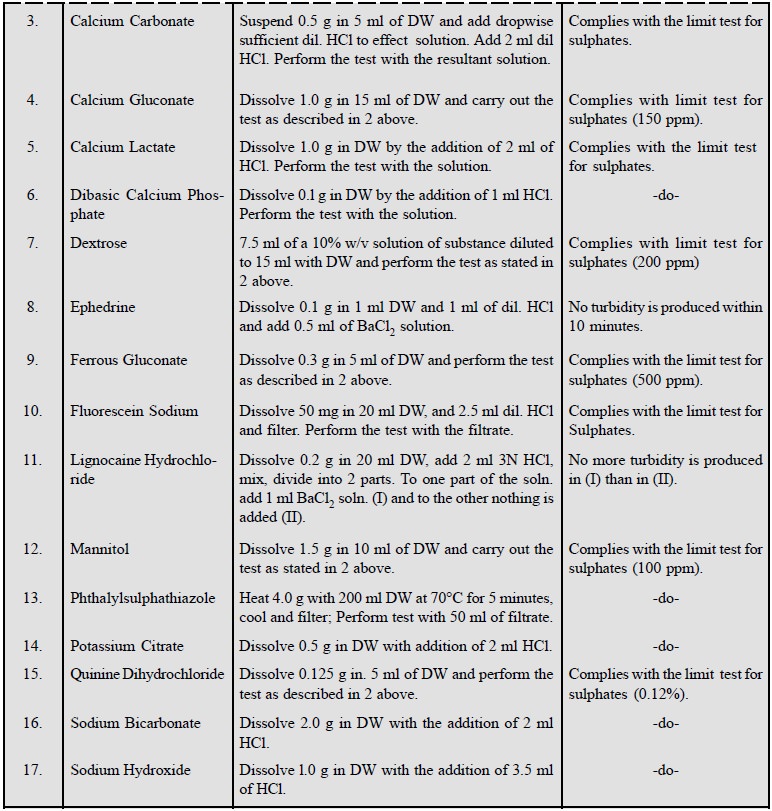
2. Limit Test for Sulphates
Theory : The limit test for sulphates
is based upon its precipitation as barium sulphate in the presence of barium chloride, hydrochloric acid and
traces of barium sulphate. In this combination, hydrochloric acid exerts its
common ion effect whereas traces of BaSO4 aids in the rapid and
complete precipitation by seeding. Thus, the opalescence caused by the sample
is compared immediately with a standard turbidity produced with a known amount
of the SO42– ion.
The main objective of this test is to provide a rigid
control of sulphate as an impurity present primarily in inorganic
pharmaceutical substances.
Materials Required : Nessler cylinders 1 pair ;
dilute hydrochloric acid (10% w/v of HCl) 2.0 ml.
Barium Sulphate Reagent : Mix 15 ml of 0.5 M barium
chloride, 55 ml of DW, and 20 ml of sulphate free alcohol, add 5 ml of a 0.0181% w/v soln. potassium sulphate
dilute to 100 ml with DW, and mix. It should always be prepared fresh.
0.5 M Barium Chloride : BaCl2 dissolved in DW to contain in 1 Litre
122.1 g of BaCl2. 2H2O.
Standard Turbidity : Place 1.0 ml of a 0.1089% w/v
soln. of K2SO4 and
2 ml of dilute HCl in a Nessler
cylinder, dilute to 45 ml with DW, add 5 ml BaSO4 reagent, stir
immediately with a glass rod and allow to stand for 5 minutes.
Procedure : Dissolve the specified
quantity of the substance in DW, transfer to a Nessler cylinder, and the preparation of the solution.
Dilute to 45 ml with DW, add 5 ml barium sulphate reagent, stir immediately
with a glass rod, and allow to stand for 5 minutes. The turbidity is not
greater than the standard turbidity, when viewed transversely.
A few examples of this test
consisting of a cross-section of pharmaceutical substances are stated below :
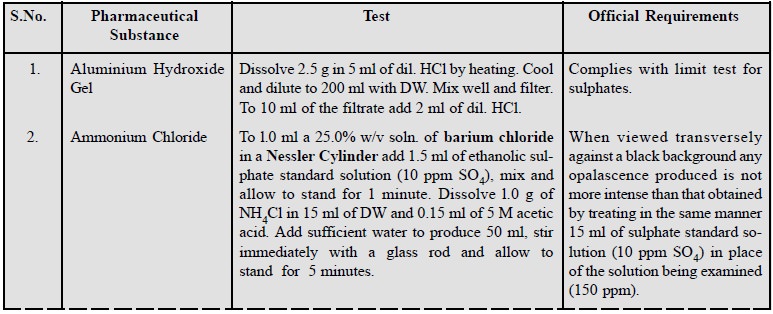
3. Limit Test for Arsenate
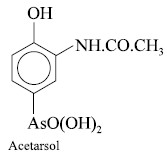
Acetarsol : An organic arsenic compound,
being therapeutically active when administered orally, that might be of value in the treatment of spirochaetal or protozoal
diseases, for instance : syphilis, yaws, relapsing fever, sleeping sickness and
amoebic dysentry.
It is made from p-hydroxyphenylarsonic acid, which may
be prepared either by straight forward meth-ods from phenol or from p-aminophenylarsonic acid. The resulting
compound obtained from either of these reactions is nitrated, reduced and the
base is finally acetylated to afford acetarsol.
Inorganic arsenates are found to be extremely
toxic in nature and hence careful control is maintained by the addition of magnesium ammonio-sulphate solution to an
aqueous solution of the sample, thereby producing an instant white precipitate.
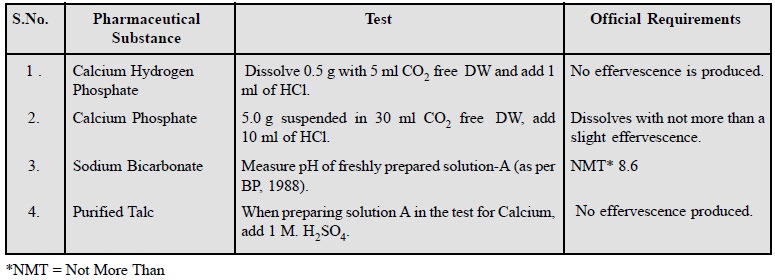
4. Limit Test for Carbonate
Carbonate impurity in pharmaceutical chemicals usually
arise from contamination with atmospheric CO2.
Examples of a few official
compounds subject to this test from the Pharmacopoeia are given below :
5. Limit Test for Cyanide
Cyanide present in Edetate Disodium is assayed by
titration with AgNO3 in neutral solution employing dimethylaminobenzylidenerhodamine
as an adsorption indicator with a colour change from yellow to orange.
A few typical examples are illustrated below :
A. Edetate Disodium
Materials Required : Edetate disodium 30.0 g ;
sodium hydroxide solution (20% w/v in DW) 35.0 ml ; dimethylaminobenzylidenerhodamine solution (0.02% w/v in acetone)
1.0 ml ; 0.01 N AgNO3 solution (1.699 g in 1 litre of DW) 100 ml.
Procedure : Dissolve 30.0 g in a mixture
of 100 ml DW and 35 ml NaOH solution, add 1 ml dimethylaminobenzylidenerhodamine and titrate with 0.01N silver
nitrate until the colour of the solution changes from yellow to orange. Repeat
the operation without the disodium edetate. The difference between the
titrations is not more than 1.25 ml.
B. Iodine
Materials Required : Iodine 3.5 g ; zinc powder 10
g ; ferrous sulphate solution (2.0% w/v in boiled and cooled DW) 1.0 ml ; sodium hydroxide solution (20% w/v in DW)
1 ml ; hydrochloric acid (~ 11.5 N) 20 ml.
![]()
Procedure : Triturate 3.5 g thoroughly
with 35 ml DW, filter and decolorise the filtrate by the addition of a little zinc powder. To 5.0 ml of
the filtrate add a few drops of ferrous sulphate solution and 1 ml NaOH
solution ; warm gently and acidify with HCl, no blue colour or green colour is
produced.
C. Potassium Iodide
Materials Required : Potassium iodide 0.5 g ;
ferrous sulphate solution (2.0% w/v in boiled and cooled DW) 1 drop ; NaOH solution (20% w/v in DW) 0.5 ml ; HCl 20.0 ml.
Procedure : Dissolve 0.5 g in 5 ml warm
DW, add 1 drop of ferrous sulphate solution and 0.5 ml NaOH solution and acidify with HCl, no blue colour is produced.
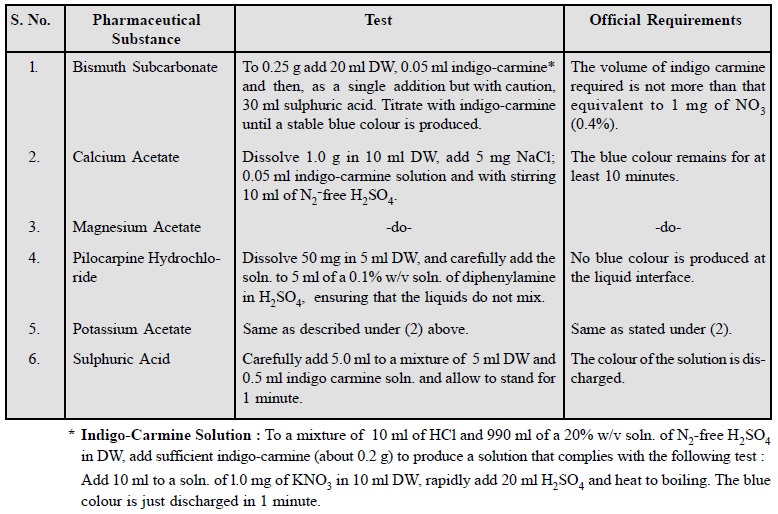
6. Limit Test for Nitrate
Basic nitrate is usually found as an impurity in bismuth
salts (e.g., bismuth subcarbonate),
very often due to the mode of preparation from the metal via bismuth nitrate.
BP (1914) first described a limit test, based upon the
production of coloured nitro-compounds by the interaction of traces of nitrates
with phenol-2, 4-disulphonic acid, and the conversion of these subsequently
into dark-yellow ammonium salts. However, this test has a serious disadvantage
of correctly matching the yellow colours with great difficulty.
BP (1932) put forward a more
reliable test for nitrate based upon the oxidation of indigocarmine to
colourless substances by the action of traces of nitrates in presence of hot
and fairly concentrated sulphuric acid, and the reaction may be expressed as
follows :
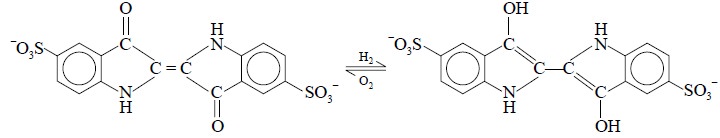
The quantities as specifed in the Pharmacopoeia allow an
official limit of nitrate equivalent to about 0.29% BiONO3.
A few typical instances of
pharmaceutical substances are enumerated below :
7. Limit Test for Oxalate
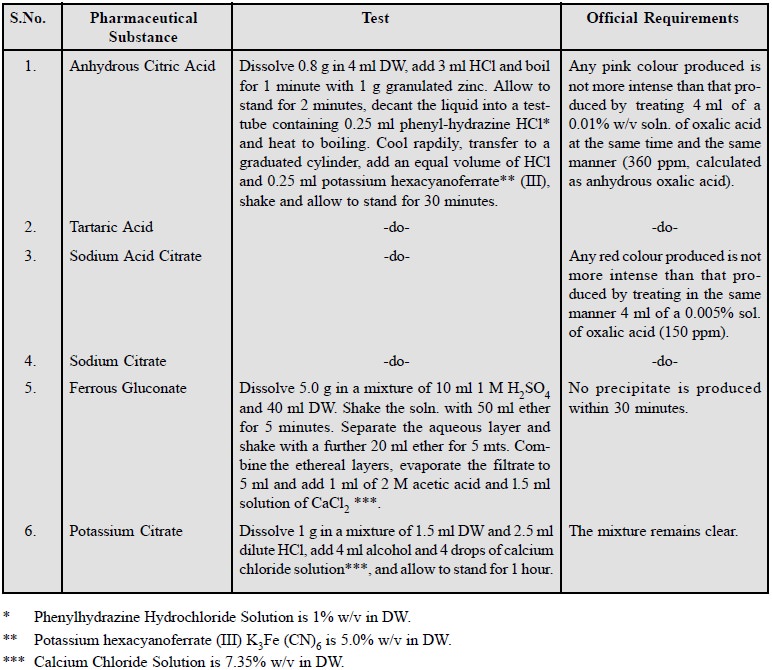
Oxalate is found to be a frequent impurity in
pharmaceutical substances belonging to the category of either organic acids e.g., anhydrous citric acid, tartaric
acid; or salts of organic acids e.g.,
ferrous gluconate, sodium citrate, potassium citrate and sodium cromoglycate.
The presence of this impurity is due to the following two prime factors, namely
:
(a) The use of
oxalic acid to get rid of Ca2+ during various manufacturing
processes.
(b) The use of
oxalic acid in the isolation and purification of organic bases e.g., ephedrine (thereby resulting into
the formation of well defined crystalline oxalates).
A few typical examples are
cited below :
8. Limit Test for Phosphate
The limit test for phosphate
is based upon the formation of a yellow colour reaction with molybdovanadic
reagent (combination of ammonium vanadate and ammonium molybdate) in an acidic
medium. However, the exact composition of the molybdovanadophosphoric acid
complex is yet to be established.
Three typical examples of pharmaceutical substances are
stated below :
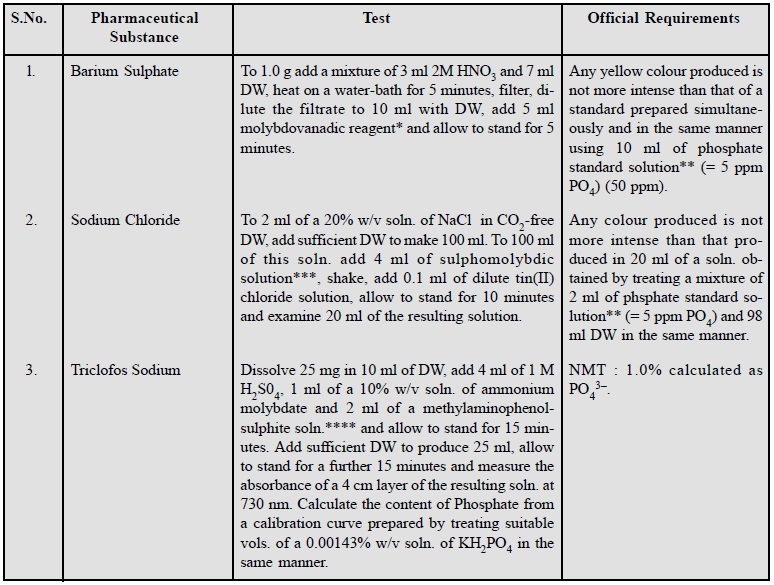
Molybdovanadic Reagent :
Suspend
4.0 g of finely powdered ammonium molybdate and 0.1 g of finely powdered ammonium metavanadate in 70 ml DW and
grind until dissolved. Add 20 ml of HNO3 and dilute to 100 ml with
DW.
Phosphate Standard
Solution (5
ppm PO4) : Dilute 0.5 ml of a 0.143% w/v soln. of potassium dihydrogen orthophosphate (KH2PO4)
to 100 ml with DW.
Sulphomolybdic Solution
: Dissolve
with heating, 25 ml ammonium molybdate in 200 ml DW. Separately, with care, add 280 ml H2SO4
to 500 ml DW. Cool and mix the two solutions and dilute to 1 Litre with DW.
Methylaminophenol-sulphite
Solution : Dissolve
0.1 g of 4-methylaminophenol sulphate, 20 g sodium metabisulphite and 0.5 g anhydrous sodium sulphite in sufficient
DW to produce 100 ml.
Related Topics