Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Recombinant Coagulation Factors and Thrombolytic Agents
Lanoteplase - Thrombolytic Agents
Lanoteplase
Lanoteplase is currently in development and published information is
limited at this time. Lanoteplase is a t-PA variant in which the fibronectin
fingerlike and epidermal growth factor domains have been removed (Collen et
al., 1988). In addition, an asparagine to glutamine substitution at amino acid
117 provides reduced clearance (Hansen et al., 1988). Lanoteplase has enhanced
fibrinolytic activity in the presence of fibrin-related plasminogen, and it is
more fibrin specific compared with streptokinase and urokinase. The
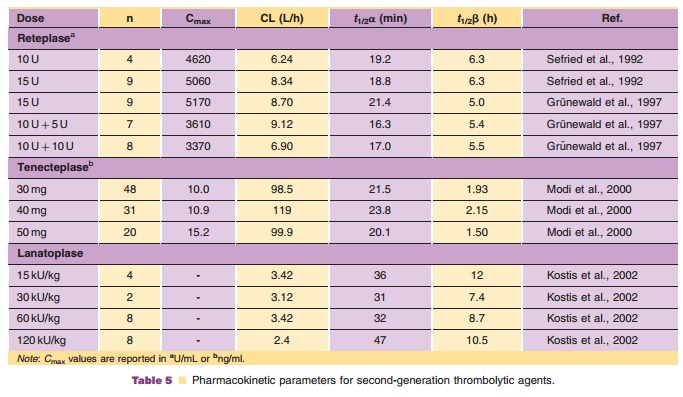
pharmacokinetics of lanoteplase in AMI patients are summarized in Table 5.
The Intravenous nPA for Treatment of Infarcting Myocardium Early
(InTIME) study was a multicenter, double-blind, randomized, double placebo,
dose-ranging study in 613 patients comparing 4 doses of lanoteplase with
accelerated alteplase. Patients were randomized to receive intravenous bolus
doses of 15, 30, 60, or 120 kU/kg (not to exceed 12,000 kU) of lanoteplase or
accelerated alteplase (den Heijer et al., 1998). A statistically significant
increase in the proportion of patients with TIMI grade 3 flow at 60 minutes was
noted with increasing lanoteplasedose (p< 0.001). Patients given the
highest lanoteplase dose appeared to have a higher rate of TIMI grade 3 flow at
90 minutes compared with alteplase (57% vs. 46%), although this may be a result
of the unusually low TIMI grade 3 flow in the alteplase arm of this small
study. There was no difference in the 30-day composite endpoint of death, heart
failure, major bleeding, or non-fatal infarction (Ross, 1999).
A larger randomized, multicenter equivalence trial (InTIME II) in 15,078
patients compared the safety and efficacy of 120 kU/kg lanoteplase with that of
accelerated alteplase (Ferguson, 1999). Patients were randomized in a 2:1
fashion to the lanoteplase arm or the alteplase arm. The primary endpoint of
the study was 30-day mortality with an incidence of 6.7% for lanoteplase and
6.6% for alteplase. The difference in the incidence of stroke was not
statistically significantly different between the treatment groups (1.89% for
lanoteplase and 1.52% for alteplase). Intracranial hemorrhage was significantly
more fre-quent in the lanoteplase arm than in the alteplase arm (1.13% vs.
0.62%).
Related Topics