Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Recombinant Coagulation Factors and Thrombolytic Agents
Recombinant Coagulation Factor IX
FACTOR IX
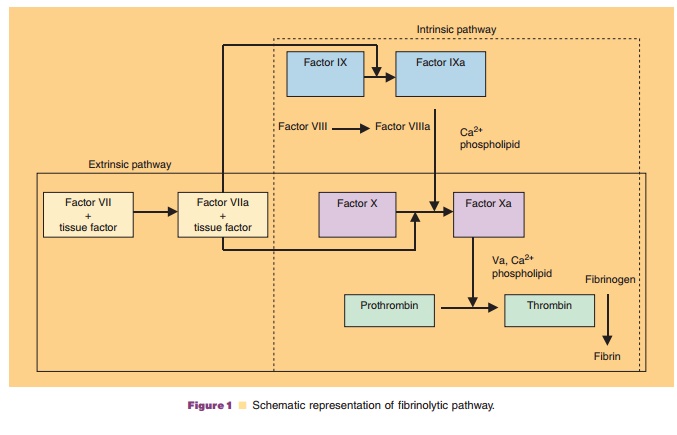
Factor IX is activated by factor VII/tissue factor complex in the extrinsic pathway and by factor XIa in the intrinsic pathway (Fig. 1). Activated factor IX, in combination with activated factor VIII, activates factor X, resulting in the conversion of prothrombin to thrombin. Thrombin then converts fibrinogen to fibrin, forming a blood clot at a site of hemorrhage.
Recombinant Factor IX
Recombinant coagulation factor IX (BeneFix , Wyeth) is produced in a CHO cell line. The transfected cell line secretes recombinant factor IX in the culture medium from which the protein is purified via several chromatographic steps. Recombinant factor IX is a 415 amino acid glycoprotein with a molecular weight of ∼55 kDa.
While plasma-derived factor IX carries a Thr/ Ala dimorphism at position 148, the primary amino acid sequence of recombinant factor IX is identical to the Ala148 allelic form. As a result of post-transla-tional modifications, recombinant and plasma-derived factor IX differ in a number of respects (White et al., 1997). First, plasma-derived factor IX carries 12 γ-carboxyglutamic acid (Gla) residues in its amino-terminal Gla-domain, whereas 40% of recombinant factor IX is undercarboxylated, lacking γ-carboxylation at Glu40. Other differences betweenrecombinant and plasma-derived factor IX are in the activation peptide region (residues 146–180), which is cleaved off upon factor IX activation. These include the lack of sulfation at Tyr155, and of phosphorylation at Ser158, as well as different N-linked glycosylation patterns at Asn157 and Asn167.
The potency of recombinant factor IX is deter-mined in IUs using an in vitro clotting assay. One international unit is the amount of factor IX activity present in a milliliter of pooled normal human plasma. The specific activity of BeneFix is greater than or equal to 200 IU/mg protein.
Pharmacology
In Beagle dogs, administration of recombinant factor IX once daily for 14 days at doses of 50, 100, or 200 IU/ kg showed a dose-proportional increase in maximum concentration (Cmax) and area under the curve (AUC). The elimination half-life ranged between 10.9 and 15.8 hours and was independent of dose (McCarthy et al., 1995). In addition, the pharmacokinetics were similar between Days 1 and 7. Pharmacokinetic and pharmacodynamic studies have indicated that in-creases in recombinant factor IX plasma concentra-tions are correlated (r¼ 0.86) with factor IX activity as measured by a clotting assay (Schaub et al., 1998). Comparison of recombinant factor IX and plasma-derived factor IX in a dog model of hemophilia B indicated that while plasma-derived factor IX had a higher AUC and Cmax compared with recombinant factor IX, the efficacy of the two products was similar (Keith et al., 1995).
Pharmaceutical Considerations
Recombinant factor IX (BeneFix ) is supplied as a sterile, non-pyrogenic, lyophilized powder in single-use vials containing nominally 250, 500, or 1000 IU per vial. The approved shelf life is 36 months at 2 C to 8 C. The product has been stored at room temperature (25 C) for up to 12 months. Freezing should be avoided to prevent damage to the diluent vial.
Recombinant factor IX should be reconstituted with the sterile water for injection (diluent) provided. After reconstitution, BeneFix should be injected intravenously over several minutes. The product does not contain a preservative and should be used within 3 hours of reconstitution.
Clinical Usage
BeneFix is indicated for the control and prevention of hemorrhagic episodes in patients with hemophilia B, including control and prevention of bleeding in surgical settings.
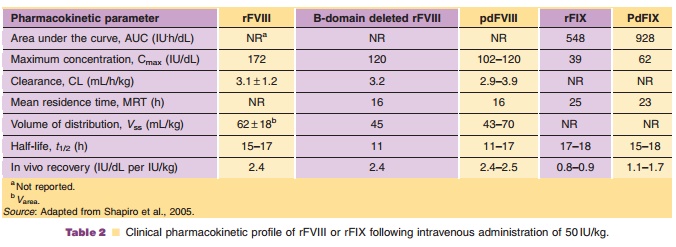
The pharmacokinetics of recombinant factor IX and of plasma-derived factor IX are summarized in Table 2. The in vivo recovery using BeneFix was 28% less than the recovery using highly purified plasma-derived factor IX, whereas there was no difference in the biological half-life. Factor IX clotting activity is highly correlated (r¼ 0.97) with plasma concentra-tions of recombinant factor IX (White et al., 1998).
The dosage and duration of substitution treat-ment depends on the severity of factor IX deficiency, the location and extent of bleeding, the clinical condition, patient age, and the desired recovery in factor IX. The dose of BeneFix should be based on the empirical finding that 1 IU of factor IX per kilogram is expected, on average, to increase the circulating level of factor IX by 0.8 ± 0.2 IU/dL (range 0.4–1.4).
Higher doses of factor IX may be necessary in patients with inhibitors. If the expected levels of factor IX are not attained, or if bleeding is not controlled, biological testing may be merited to determine if factor IX inhibitors are present.
Safety
Since BeneFix is produced in a CHO cell line, it may be contraindicated in patients with known history of hypersensitivity to hamster protein and other consti-tuents in the preparation. During uncontrolled open-label clinical studies with recombinant factor VII, adverse events reported in more than 2% of patients included nausea, taste perversion, injection site reac-tion, injection site pain, headache, dizziness, allergic rhinitis, rash, hives, flushing, fever, and shaking.
Related Topics