Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Recombinant Coagulation Factors and Thrombolytic Agents
Recombinant Coagulation Factor VIIA
FACTOR VIIA
Development of recombinant factor VIIa was motivated by the fact that a small fraction of patients with hemophilia (15–20% of patients with hemophilia A and 2–5% of patients with hemophilia B) develop antibodies (inhibitors) to factor VIII or factor IX. High titers of inhibitors make it impossible to give sufficient coagulation factor to overcome the inhibitor, and therapy is ineffective or is associated with unacceptable side effects. Factor VIIa can be valuable in these instances since in the absence of tissue factor, factor VII has very low proteolytic activity.
Structure
Factor VII is a vitamin K-dependent glycosylated serine protease proenzyme that is synthesized in the liver. It has 406 amino acids and the molecular weightis ∼50 kDa. The protein is not functionally active unless it is γ-carboxylated. There are two sites of N-linked glycosylation on factor VII. Factor VII is synthesized as a proenzyme that becomes activated and cleaved upon hydrolysis of Arg-152 and Ile-153.
Recombinant Factor VIIa
Recombinant factor VII is expressed in baby hamster kidney cells as a single-chain form and is spontaneously activated to factor VIIa during purification. Characterization of the protein indicates that it is very similar to plasma-derived factor VIIa with regard to amino acid sequence, carbohydrate com-position, and γ-carboxylation (Thim et al., 1988).
Pharmacokinetics and Pharmacodynamics
The single-dose pharmacokinetics of recombinant factor VIIa were investigated in 15 patients with hemophilia with severe factor VIII or factor IX deficiency (Lindley et al., 1994). Table 3 summarizes the pharmacokinetics from this study. Following an intravenous dose of 17.5, 35, and 70 µg/kg, the plasma clearance was 30.3, 32.5, and 36.1 mL/h/kg, respectively. The pharmacokinetics were linear and no difference in clearance was noted between non-bleeding and bleeding episodes. Median clearance was 31.0 mL/h in non-bleeding episodes and 32.6 mL/h in bleeding episodes. The median half-life was 2.9 hours in non-bleeding episodes and 2.3 hours in bleeding episodes. There was considerable inter-individual variability whereas intraindividual variability appears to be lower.
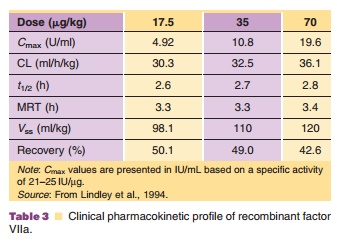
Based on preclinical studies in dogs, a concen-tration of > 8 U/mL should result in immediate hemostasis, whereas a concentration of 4 U/mL seems to be below the hemostatic level (Hedner, 1996). Based on the results of several pharmacokinetic/pharmaco-dynamics studies, it is necessary to maintain plasma levels above 5 to 6 U/mL for adequate hemostasis. This may be done by administering sufficiently high initial concentrations to ensure this, or by maintaining a strict 2-hour dosing interval following doses of 70 to 90 µg/kg (Hedner, 1996).
Pharmaceutical Considerations
NovoSeven (Novo Nordisk) is supplied as a white lyophilized powder in single-use glass vials formulated with sodium chloride, calcium chloride dihy-drate, glycylglycine, polysorbate 80, and mannitol. The pH is adjusted to 5.3 to 6.3. The product does not contain any stabilizing protein. Before reconstitution, NovoSeven should be stored refrigerated (2–8 C/ 36–46 F) avoiding exposure to direct sunlight.
NovoSeven should be reconstituted with sterile water for injection, USP (2.2 mL for the 1.2-mg vial and 8.5 mL for the 4.8-mg vial). After reconstitution with the appropriate volume of diluent, each vial contains approximately 0.6 mg/mL. Following reconstitution, NovoSeven may be stored refrigerated or at room temperature for up to 3 hours. NovoSeven is intended for intravenous bolus injection and should not be mixed with infusion solutions.
Clinical Usage
Recombinant factor VIIa (NovoSeven ) is indicated for the treatment of bleeding episodes or the prevention of bleeding in surgical intervention or invasive procedures in patients with hemophilia A or B with inhibitors to factor VIII or factor IX. It is also indicated for treatment of bleeding episodes or the prevention of bleeding in surgical intervention or invasive procedures in patients with congenital factor VII deficiency. The recommended dose of recombinant factor VIIa for patients with hemophilia A or B with inhibitors is 90 µg/kg every 2 hours by bolus infusion until hemostasis is achieved or until treatment is judged to be inadequate. The minimum effective dose has not been established. Doses between 35 and 120 µg/kg have been used successfully in clinical trials. Both the dose and administration interval maybe adjusted based on the severity and degree of hemostasis.
A randomized, double-blind study investigating 35 and 70 µg/kg recombinant factor VIIa in 84 patients with hemophilia A or B with or without inhibitors found both doses were ∼70% effective (Lusher et al., 1998a). A randomized study evaluating doses of 35 and 90 µg/kg in hemophilia patients with inhibitors undergoing elective surgery showed that the higher dose was more effective than the lower dose (Shapiro et al., 1988).
Safety
Based on the clinical safety database of 1939 treatment episodes in 298 patients with hemophilia A or B with inhibitors, adverse events reported at rates of more than 2% of patients treated included fever, hemorrhage, decreased fibrinogen, hemarthrosis, and hypertension.
Recombinant factor VIIa (NovoSeven ) should not be administered to patients with known hyper-sensitivity to recombinant factor VIIa or any of the components of recombinant factor VIIa. Recombinant factor VIIa is contraindicated in patients with known hypersensitivity to mouse, hamster, or bovine proteins.
Related Topics