Chapter: Biotechnology Applying the Genetic Revolution: Recombinant Proteins
Improving Protein Secretion
IMPROVING
PROTEIN SECRETION
When a bacterial cell
synthesizes a recombinant protein, it may end up in the cytoplasm, the
periplasmic space between the inner and outer membranes, or be exported out of
the cell into the culture medium. Secretion across the inner, cytoplasmic,
membrane is directed by the presence of a hydrophobic signal sequence at the
N-terminal end of the newly synthesized protein. The signal sequence is cut off
after export by signal peptidase (also known as leader peptidase). Although
bacteria such as E. coli export few
proteins, special secretion systems do exist that allow export of proteins
across both membranes into the culture medium.
It is obviously easier to
purify a secreted protein than one that remains in the cytoplasm with the
majority of the bacterial cell’s own proteins. On the other hand, many proteins
are unstable when outside the cellular environment and/or exposed to air.
Generally, such proteins are not used in biotechnology since they are awkward
to purify or to use. Most proteins and enzymes chosen for practical use are
relatively stable outside the cell. A standard goal is to arrange for them to
be secreted in order to help isolation and purification. Several approaches
exist for this:
(a)
A signal sequence is engineered into the cloned gene. Consequently,
the recombinant protein will have an
N-terminal signal sequence when newly synthesized. This will direct its export
to the periplasm by the general
secretory system. The bacterial cells are then harvested and treated so as
to permeabilize or remove the outer membrane, thus releasing the recombinant
protein.
One major problem is that
large amounts of a recombinant protein may overload the secretory machinery and
aggregate into inclusion bodies. Substantial amounts of the recombinant protein
may accumulate inside the cell with the signal sequence still attached.
Secretion may sometimes be increased dramatically by providing elevated levels
of the secretory system proteins (Sec proteins). Extra copies of the sec genes
can be cloned and expressed on plasmids to increase the protein amounts. Mutant
E. coli strains also have been
developed that facilitate secretion.
(b)
The recombinant protein may be fused to a bacterial protein that is
normally exported (see the following section on fusion vectors). The maltose
binding protein of E. coli is
efficiently exported to the periplasmic space and is a favorite carrier for
recombinant proteins. The recombinant protein is later released from the
carrier protein by protease cleavage. This technique is especially useful for
relatively short peptides, including many hormones and growth factors, which
are often unstable alone.
(c)
The gene of interest can be expressed in gram-positive bacteria,
such as Bacillus, which lack an outer
membrane. Consequently, exported proteins go out into the culture medium.
Although this is convenient, the genetics of Bacillus is still far behind that of E. coli. Animal cells are another alternative as they have only a
single cytoplasmic membrane, and therefore secrete proteins directly into the
medium.
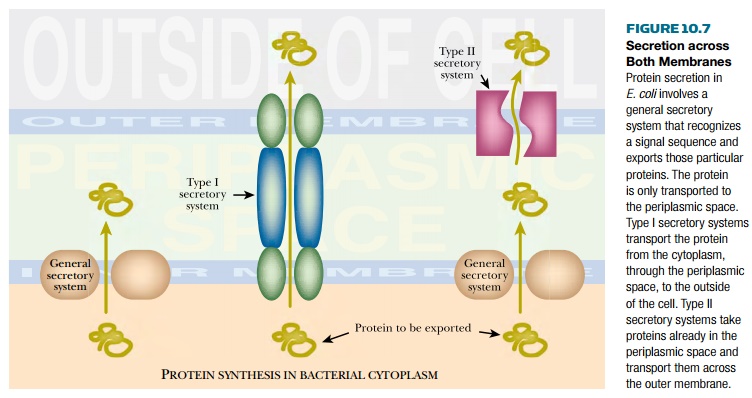
(d) Secretion across both membranes of gram-negative bacteria such as E. coli may be achieved by specialized export systems (Fig. 10.7). Most of these export systems are used naturally for the secretion of toxins by pathogenic strains of bacteria. Thus the type I secretory system spans both inner and outer membranes and is used to secrete hemolysin by some E. coli strains that cause urinary infections. The type II secretory system spans the outer membrane only. Export into the medium therefore requires the general secretory system (i.e., the Sec system) to export the protein across the inner membrane first. Normally, this two-step process is used by Pseudomonas to secrete exotoxin A.
Strains of E.
coli engineered to deploy these alternative export systems are beginning to
be used. In these cases it is necessary to modify the protein to be exported so
that it is recognized by the chosen export system. Engineering the gene with
the appropriate signal sequences allows the protein to be recognized and
exported.
Related Topics