Chapter: Biotechnology Applying the Genetic Revolution: Recombinant Proteins
Expression of Proteins by Yeast
EXPRESSION OF PROTEINS BY YEAST
As already discussed, brewer’s yeast,
Saccharomyces cerevisiae, has been used as a model single- celled eukaryote in
molecular biology. The yeast genome has been sequenced and many genes have been
characterized. From the viewpoint of biotechnology, several other factors favor
the use of yeast for production of recombinant proteins:
(a)
Yeast can be grown easily on both a small scale or in large
bioreactors.
(b)
After many years of use in brewing and baking, yeast is accepted as
a safe organism. It can therefore be used to produce pharmaceuticals for use in
humans without needing extra government approval.
(c)
Yeast normally secretes very few proteins. Consequently, if it is
engineered to release a recombinant protein into the culture medium, this can
be purified relatively easily.
(d)
DNA may be transformed into yeast cells either after degrading the
cell wall chemically or enzymatically, or, more usually, by electroporation.
(e)
A naturally occurring plasmid, the 2-micron circle, is available as
a starting point for developing cloning plasmids.
(f)
A variety of yeast promoters have been characterized that are
suitable for driving expression of cloned genes.
(g)
Although only a primitive single-celled organism, yeast nonetheless
carries out many of the posttranslational modifications typical of eukaryotic
cells, such as addition of sugar residues (glycosylation). However, yeast only
glycosylates proteins that are secreted.
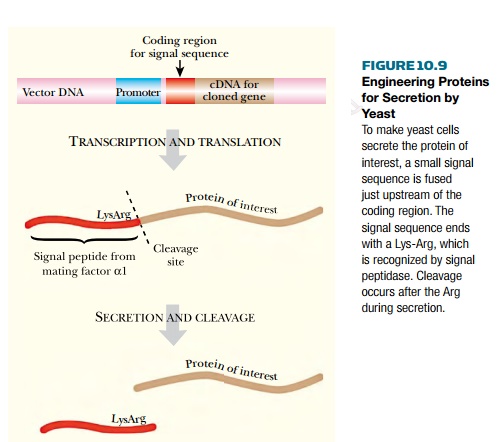
Recombinant proteins may be engineered for
secretion by yeast (Fig. 10.9). Adding a signal peptide upstream of the coding
region flags the protein for secretion. The signal sequence used is taken from
the gene for mating factor α, a protein that is normally
secreted. Yeast signal peptidase recognizes the sequence Lys-Arg and cleaves
off the signal peptide once the protein has crossed the cell membrane. It is
therefore necessary to position the two codons for Lys-Arg immediately upstream
of the coding sequence for the recombinant protein to ensure that the engineered
protein has a correct N-terminal sequence.
A variety of vectors exist for use with yeast.
These may be classified into three main classes:
(a)
Plasmid vectors are used most often. These are typically yeast
shuttle vectors that can replicate in E.
coli as well as yeast. The yeast
replication system is normally derived from the yeast 2-micron circle.
(b)
Vectors that integrate into the yeast chromosomes. Because plasmids
may be lost, especially in large-scale cultures, this approach has the advantage
of stability. The disadvantage is that only a single copy of the cloned gene is
present, unless tandem repeats are used. These, however, are unstable because
of crossing over. In practice this approach is rarely used.
(c)
Yeast artificial chromosomes. These are used for cloning and
analysis of large regions of eukaryotic genomes. However, they are not
convenient for use as expression vectors.
Assorted proteins for human
use are now produced using Saccharomyces cerevisiae. These include insulin,
factor XIIIa (for blood coagulation), several growth factors, and several virus
proteins (from human immunodeficiency virus, hepatitis B, hepatitis C, etc.)
used as vaccines or in diagnostics.
Although many recombinant
proteins have been expressed successfully in yeast, yields are often low. The
problems include:
(a)
Loss of expression plasmids during growth in large bioreactors.
(b)
Proteins supposed to be secreted are often retained in the space
between membrane and wall rather than exiting into the culture medium.
(c)
Glycosylation of proteins is often excessive and the final protein
may have too many sugar residues attached to function correctly.
Related Topics