Chapter: Physics : Quantum Physics
Important Short Question and Answers: Quantum Physics
1. Explain Planck’s hypothesis or what the postulates of Planck’s quantum theory?
Or what are the assumptions of quantum theory of black body radiation?
a. The electrons in the black body are assumed as simple harmonic oscillators.
b. The oscillators will not emit energy continuously.
c. They emit radiation in terms of quanta of magnitude ‘hγ’, discretely.
E = nhγ where n= 1, 2, 3, 3,…..
2. What is Compton wavelength? Give its value.
The shift in wavelength corresponding to the scattering angle of 900 is called Compton wavelength.
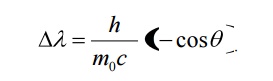
3. What is physical significance of wave function?
a. The probability of finding a particle in space, at any given instant of time is characterized by a function Ψ(x, y, z) called wave-function.
b. It relates the particle and the wave statistically.
c. It gives the information about the particle behavior.
d. It is a complex quantity.
e. |Ψ 2| Represents the probability density of the particle, which is real and positive.
3. What do you understand by the term wave function?
Wave function Ψ is a variable quantity that is associated with a moving particle at any position (x, y, z) and at any time‘t’. It relates the probability of finding the particle at that point and at that time.

5. Define Eigen value and Eigen function.
Eigen value is defined as energy of the particle and is denoted by the letter En. Eigen function is defined as the wave function of the particle and is denoted
by the letter
5. What is meant by degenerate and non-degenerate state? Give examples.
Degenerate state: for various combinations of quantum numbers if we get same Eigen value (Energy levels) but different Eigen functions, then it is called degenerate state.
Non- degenerate state: for various combinations of quantum numbers if we get same Eigen values (Energy levels) and same Eigen functions, then it is called Non- Degenerate state.
7. Mention the applications of electron microscope.
a. It has a very wide area of applications in the field of biology, metallurgy, physics, chemistry, medicine, and engineering.
b. It is used to determine the complicated structures of crystals.
c. It is used in the study of celluloids.
d. It is used to study the structure of microorganisms such as virus, bacteria etc.
8. What is meant by energy spectrum of a black body? What do you infer from it? The distribution of energy for various wavelengths at various temperatures is
known as energy spectrum of a black body.
INFERENCE:
a. When temperature increases, the wavelength decreases.
b. The total energy emitted at any particular temperature can be found with the help of the area traced by the curve.
9. Define Stefan-Boltzmann’s law.
Stefan- Boltzmann Law
According to this law the radiant energy (E) of the body is directly proportional to the fourth power of the temperature (T) of the body.
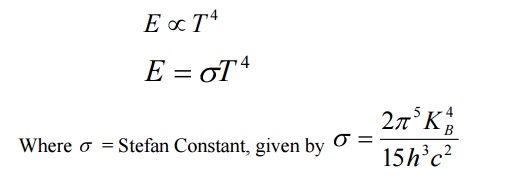
10 Define Wien’s displacement law.
Wien’s displacement law:
This law states that the product of then wavelength λ maximum energy and the absolute temperature (T) is a constant.
λmT = Cons tant
This law shows that, as the temperature increases, the wavelength corresponding to maximum energy decreases.
Wien also showed that the maximum energy (Emax) is directly proportional to the fifth power of the absolute temperature.
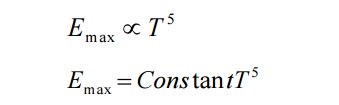
By deducing this law he obtained a law called Wien’s law of distribution of energy (Eλ), given by
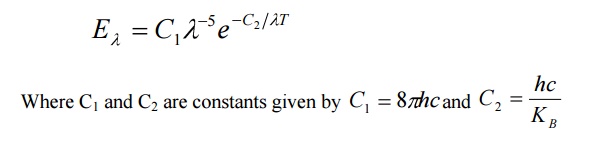
11. Define Raleigh- Jeans law.
Raleigh Jeans law:
According to this law, the energy distribution is directly proportional to the absolute temperature and is inversely proportional to the fourth power of the wavelength.
It is governed by the equation
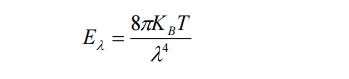
Where KB is Boltzmann Constant.
12.What are the applications of Schroedinger wave equations?
1. It is used to find the electrons in the metal
2. It is used to find the energy levels of an electron in an infinite deep potential well.
13.What is black body and what are its characteristics?
A perfect black body is the one which absorbs and also emits the radiations completely.
In practice no body is perfectly black. We have to coat the black color over the surface to make a black body.
Black body is said to be a perfect absorber, since it absorbs all the wavelengths of the incident radiation. The black body is a perfect radiator, because it radiates the entire wavelength absorbed by it. This phenomenon is called black body radiation
13. Write down the one dimensional scherodinger time independent equation and write the same for a free particle.
The one dimensional schroedinger time independent wave equation is given By
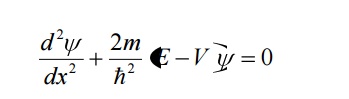
For a free particle, the potential energy is zero i.e., V = 0. Therefore the Schrodinger equation becomes
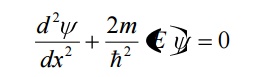
15. define normalization process and write down the normalized wave function for an electron in a one dimensional potential well of length’ a’ meters.
Normalization is the process by which the probability of finding a particle inside any potential well can be done.For a one dimensional potential well of length’ a’ meter the normalized function is given by
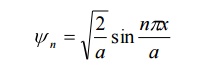
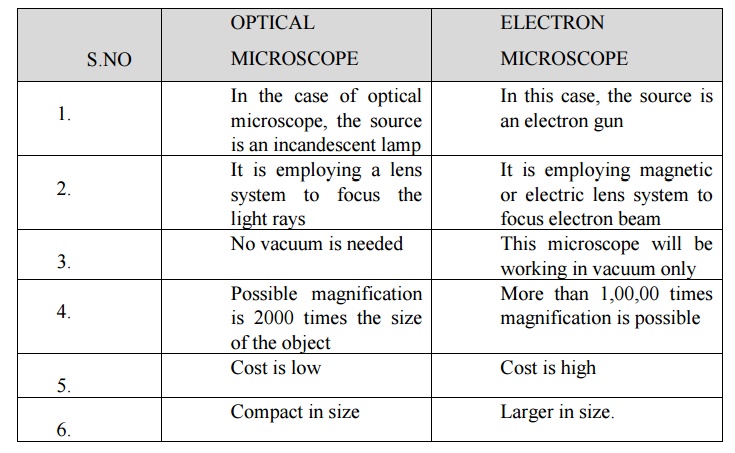
16. Differentiate between Optical Microscope and electron microscope.
Related Topics