Chapter: Biochemistry: Integration of Metabolism: Cellular Signaling
How do second messengers work? Cyclic AMP and G Proteins
How do second messengers work?
Cyclic AMP and G Proteins
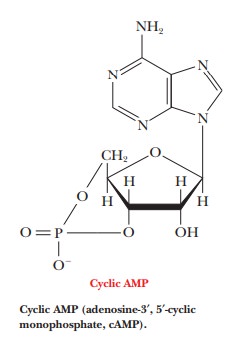
Cyclic
AMP (adenosine-3',5'-monophosphate, cAMP) is one example of a second messenger.
The mode of action starts with binding of a hormone to a specific receptor
called a β1- or β2-adrenergic receptor, which triggers the production
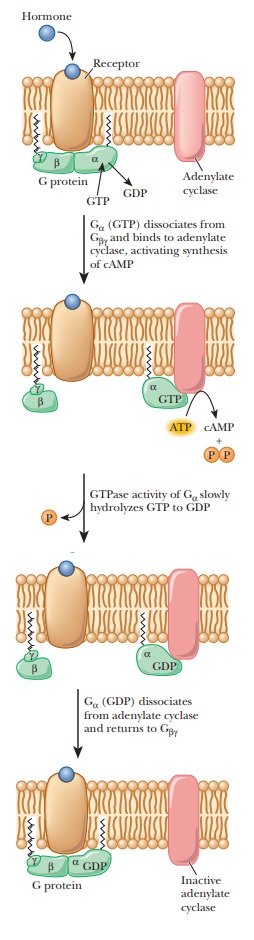

This reaction is mediated by a stimulatory G protein, a trimer consisting of
three subunits—α, β, and γ. Binding of the hormone to the receptor activates
the G protein; the α-subunit binds GTP while releasing GDP, giving
rise to the name of the protein. The active protein has GTPase activity and
slowly hydrolyzes GTP, returning the G protein to the inactive state. GDP
remains bound to the α-subunit and must be exchanged for GTP when the
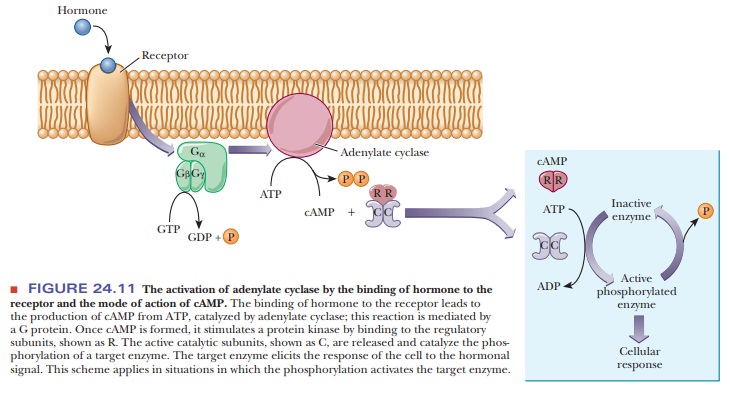
protein is activated the next time (Figure 24.9). The G protein and adenylate
cyclase are bound to the plasma membrane, while cAMP is released into the
interior of the cell to act as a second messenger. As we have already seen in
several pathways, cAMP stimulates protein kinase A, which phosphorylates a host
of enzymes and transcription factors. Some examples are known in which the
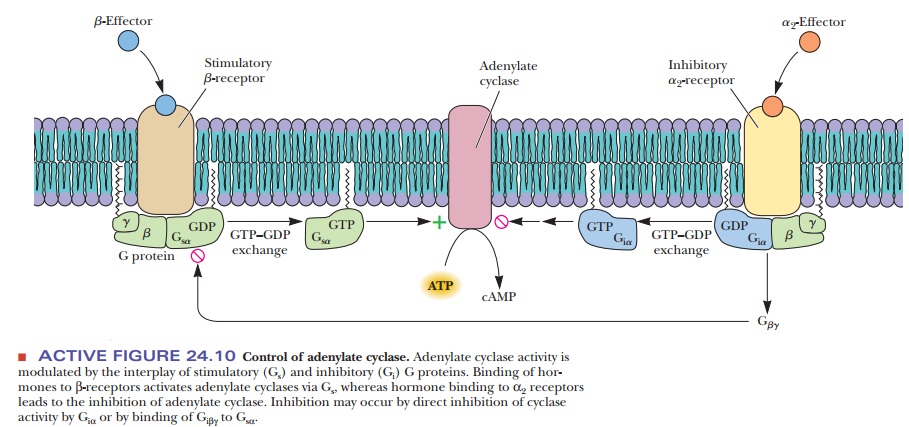
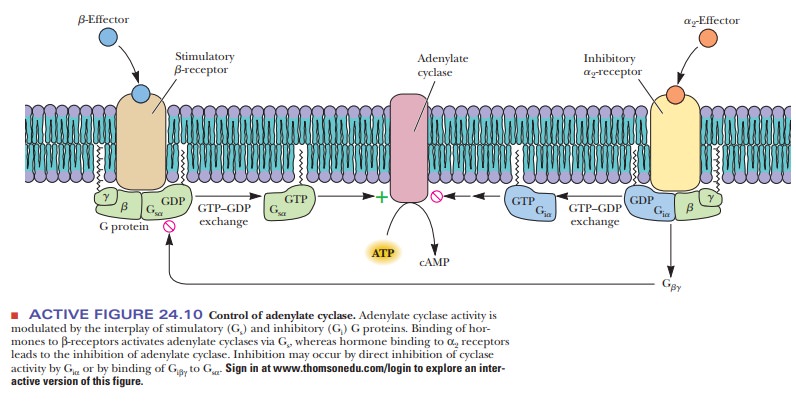
binding of hormone to receptor (anα2-receptor) inhibits rather than stimulates
adenylate cyclase. A G protein with a different kind of α-subunit mediates the process. The modified G protein is referred
to as aninhibitory G protein to
distinguish it from the kind that stimulates response tohormone binding (Figure
24.10).
In
eukaryotic cells, the usual mode of action of cAMP is to stimulate a
cAMP-dependent protein kinase, a tetramer consisting of two regulatory subunits
and two catalytic subunits. When cAMP binds to the dimer of regulatory
subunits, the two active catalytic subunits are released. The active kinase
catalyzes the phosphorylation of a target enzyme or transcription factor
(Figure 24.11). In the scheme shown in Figure 24.11, phosphorylation activates
the enzyme. Cases are also known in which phosphorylation inactivates a target
enzyme. The usual site of phosphorylation is the hydroxyl group of a serine or
a threonine. ATP is the source of the phosphate group that is trans-ferred to
the enzyme. The target enzyme then elicits the cellular response.
G proteins are very important signaling molecules in eukaryotes. They can be activated by combinations of hormones. For example, both epinephrine and glucagon act via a stimulatory G protein in liver cells. The effect can be cumulative, so that if both glucagons and epinephrine have been released, the cellular effect is greater. Besides the effect on cAMP, G proteins are involved in activating many other cellular processes, including stimulating phospholipase C and opening or closing membrane ion channels.
They are also involved in vision and smell. There are currently more than 100 known G
protein–coupled receptors and more than 20 known G proteins.
A G protein is permanently activated by cholera toxin, leading to excessive
stimulation of adenylate cyclase and chronic elevation of cAMP levels. The main
danger in cholera, caused by the
bacterium Vibrio cholerae, is severe
dehy-dration as a result of diarrhea. The unregulated activity of adenylate
cyclase in epithelial cells leads to the diarrhea because cAMP in epithelial
cells stimulates active transport of Na+.
Excessive cAMP in the epithelial cells of the small intes-tine produces a large
flow of Na+ and water from the mucosal surface of the epithelial cells into the
lumen of the intestine. If the lost fluid and salts can be replaced in cholera
victims, the immune system can eliminate the actual infec-tion within a few
days.
Related Topics