Chapter: Biochemistry: Integration of Metabolism: Cellular Signaling
Hormones and the Control of Metabolism
Hormones and the Control of
Metabolism
Now that we know something about the effects of hormones in
triggering responses within the cell, we can return to and expand on some
earlier points about metabolic control. We discussed some points about control
mechanisms in carbohydrate metabolism. We saw at that time how glycolysis and
gluconeogenesis can be regulated and how glycogen synthesis and breakdown can
respond to the body’s needs. Phosphorylation and dephosphorylation of the
appropriate enzymes played a large role there, and that whole scheme is subject
to hormonal action.
What hormones control carbohydrate metabolism?
Three hormones play a part in the regulation of carbohydrate
metabolism: epinephrine, glucagon, and insulin. Epinephrine acts on muscle
tissue to raise levels of glucose on demand, while glucagon acts on the liver,
also to increase the availability of glucose. Feedback control plays a role in
the process and ensures that the amount of glucose made available does not
reach an excessive level. The role of insulin is to trigger the feedback
response that achieves this further control.
Epinephrine (also called adrenalin)
is structurally related to the amino acid tyrosine. Epinephrine is released
from the adrenal glands in response to stress (the “fight or flight” response).
When it binds to specific receptors, it sets off a chain of events that leads
to increased levels of glucose in the blood, increased glycolysis in muscle
cells, and increased breakdown of fatty acids for energy. Glucagon (a peptide
that contains 29 amino acid residues) is released by the α-cells of the islets of Langerhans in the
pancreas, and it too binds to specificreceptors to set off a chain of events to
make glucose available to the organ-ism. Each time a single hormone molecule,
whether epinephrine or glucagon, binds to its specific receptor, it activates a
number of stimulatory G proteins. This effect starts an amplification of the
hormonal signal. Each active G protein in turn stimulates adenylate cyclase
several times before the G protein is inacti-vated by its own GTPase activity,
leading to still more amplification. The cAMP produced by the increased
adenylate cyclase activity allows for increased activity of the cAMP-dependent protein
kinase, phosphorylating target enzymes that lead to increased glucose levels.
In particular, this means an increase in the activity of the enzymes involved
in gluconeogenesis and glycogen breakdown as well as a decrease in the activity
of enzymes involved in glycolysis and glycogen synthesis. The series of
amplifying steps is called a cascade,
and the cumulative effect is the underlying reason why small amounts of
hormones can have such marked effects.
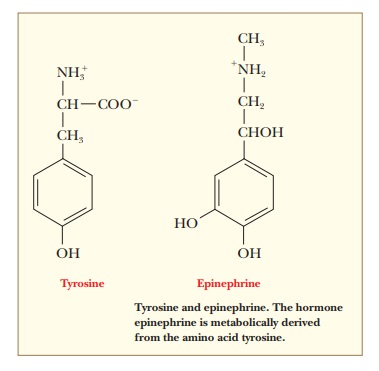
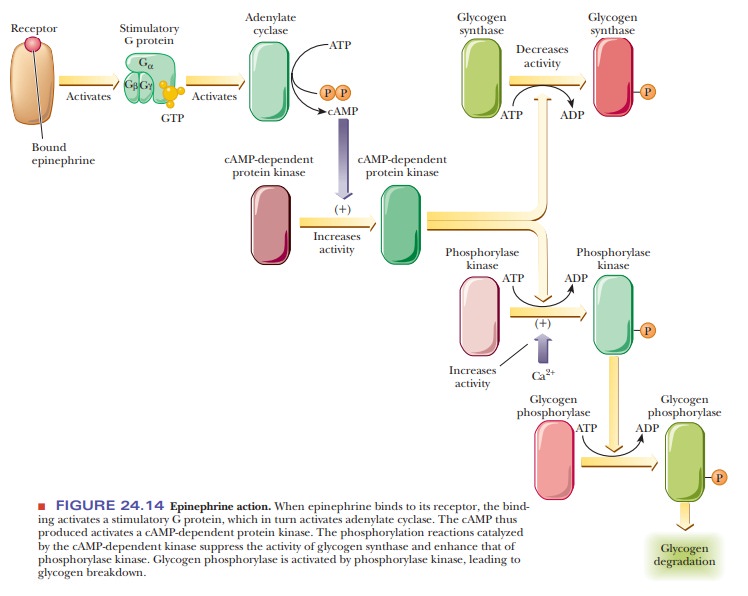
Figure 24.14 shows how the binding of epinephrine to specific
receptors leads to increased glycogen breakdown in muscle and suppression of
glycogen synthesis. The hormonal stimulation leads to activation of adenylate
cyclase, which in turn activates the cAMP-dependent protein kinase responsible
for activating glycogen phosphorylase and inactivating glycogen synthase.
The effect of glucagon binding to receptors in stimulating
gluconeogenesis in the liver and suppressing glycolysis depends on changes in
the concentra-tion of the key allosteric effector, fructose-2,6-bisphosphate (F2,6P). Recall that this
compound is an important allosteric activator of phosphofructokinase, the key
enzyme of glycolysis; it is also an inhibitor of fructose-bisphosphate phosphatase, which plays a role in gluconeogenesis. A
high concentration of F2,6P stimulates glycolysis, whereas a low concentration
stimulates gluconeogenesis. The concentration of F2,6P in a cell depends on the
balance between its synthesis [catalyzed by phosphofructokinase-2 (PFK-2)] and
its breakdown [catalyzed by fructose-bisphosphatase-2
(FBPase-2)].
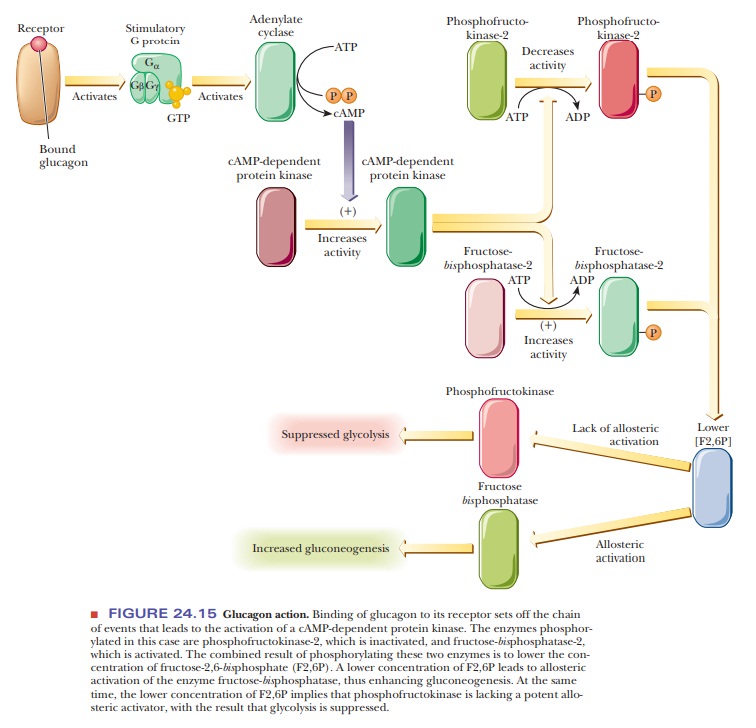
The enzyme activities (on a single multifunctional protein) that control the formation and breakdown of F2,6P are themselves controlled by a phosphorylation/dephosphorylation mechanism, which in turn is subject to the
same sort of hormonal control we just discussed for the enzymes of glycogen
metabolism. Figure 24.15 summarizes the chain of events that leads to increased
gluconeo-genesis in the liver as a result of the binding of glucagon to its
specific receptor. The following Biochemical Connections box discusses the role
of insulin in overall metabolism and some current dieting trends.
Summary
When a hormone binds to its receptor on the plasma membrane of a
tar-get cell, it sets off a cascade of reactions by which second messengers
elicit the actual cellular response.
Two of the most important second messengers, cyclic AMP (cAMP) and
phosphatidylinositol-4,5-bisphosphate
(PIP2), activate protein kinases, which phosphorylate key enzymes.
Calcium ion is intimately involved in the action of PIP2.
Epinephrine stimulates adenylate cyclase in muscle cells, leading
to activa-tion of cAMP-dependent protein kinase. This ultimately leads to
activation of glycogen phosphorylase and degradation of glycogen for energy.
Glucagon stimulates adenylate cyclase in liver cells, leading to
activation of cAMP-dependent protein kinase. This leads to inhibition of
phospho-fructokinase-2 and activation of fructose bisphosphatase-2. This lowers the level of fructose-2,6-bisphosphate, which suppresses
glycolysis and stimulates gluconeogenesis in the liver, leading to increased
glucose production.
Hormonal triggering can be added to other levels of control of
metabo-lism, such as allosteric activation and covalent modification, to ensure
an efficient response to the needs of the organism.
Related Topics