Chapter: Biochemistry: Integration of Metabolism: Cellular Signaling
Hormones and Second Messengers
Hormones and Second Messengers
Hormones
The metabolic processes within a given cell are frequently regulated by signals from outside the cell. A usual means of intercellular communication takes place through the workings of the endocrine system, in which the ductless glands produce hormones as intercellular messengers.
What are hormones?
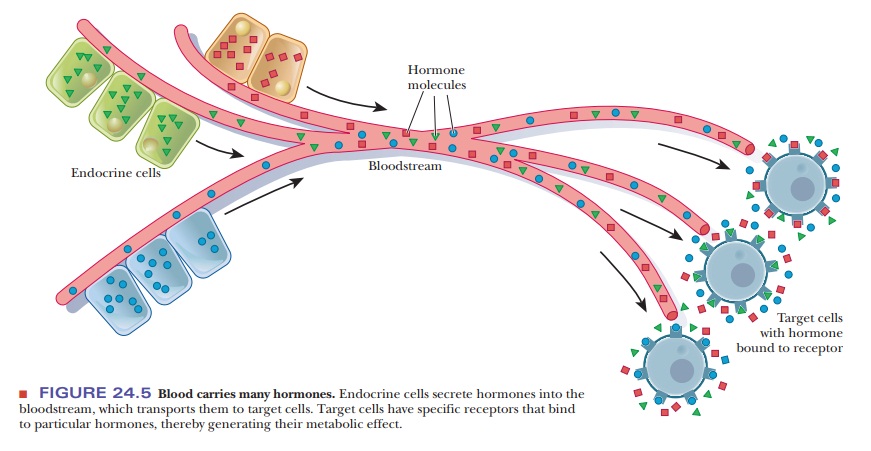
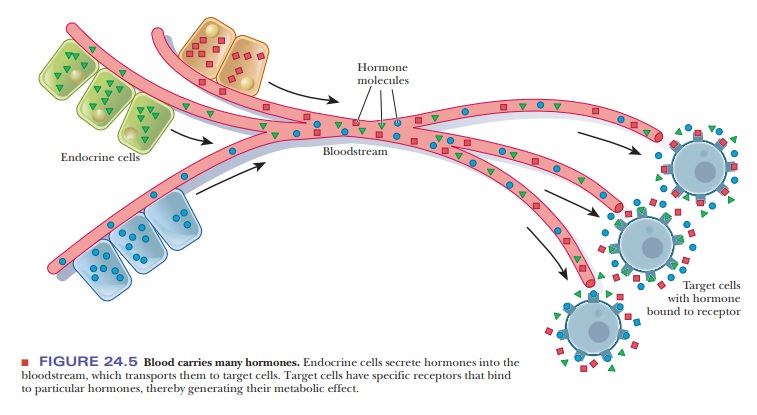
Hormones are transported from the sites of their synthesis to the
sites of action by the bloodstream (Figure 24.5). In terms of their chemical
structure, some typical hormones are steroids, such as estrogens, androgens,
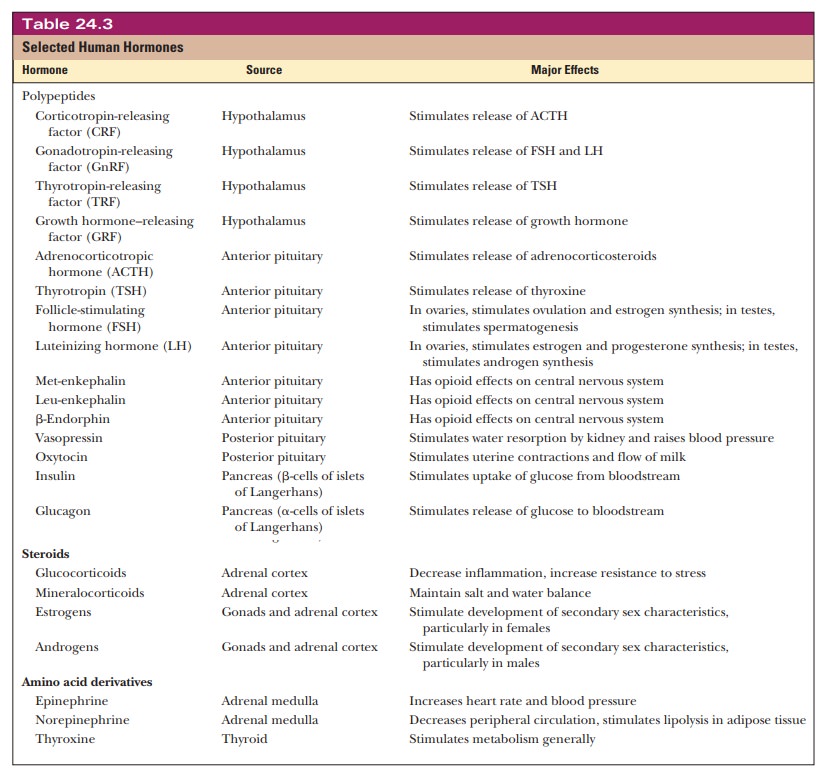
and mineralocorticoids; polypeptides, such as insulin and endorphins; and amino
acid derivatives, such as epinephrine and norepinephrine (Table 24.3).
Hormones have several important functions in the body. They help
main-tain homeostasis, the balance
of biological activities in the body. The effect of insulin in keeping the
blood glucose level within narrow limits is an example of this function. The
operation of epinephrine and norepinephrine in the “fight-or-flight” response
is an example of the way in which hormones medi-ate responses to external
stimuli. Finally, hormones play roles in growth and
The methods and insights of biochemistry and physiology alike have
helped illuminate the workings of the endocrine system.
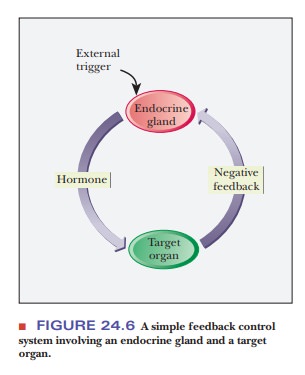
The release of hormones exerts control on the cells of target organs;
other control mechanisms, however, determine the workings of the endocrine
gland that releases the hormone in question. Simple feedback mechanisms, in
which the action of the hormone leads to feedback inhibition of the release of
hor-mone, can be postulated (Figure 24.6). The workings of the endocrine system
are, in fact, much less simple, with the added complexity allowing for a
greater degree of control. To illustrate with a rather restricted example,
insulin is released in response to a rapid rise in the level of blood glucose.
In the absence of control mechanisms, an excess of insulin can produce hypoglycemia, the condition of low
blood glucose. In addition to negative feedback control on the release of
insulin, the action of the hormone glucagon tends to increase the level of
glucose in the bloodstream. The two hormones together regulate blood glucose.
A more sophisticated control system involves the action of the hypothalamus, the pituitary, and specific endocrine
glands (Figure 24.7). The central nervous system sends a signal to the
hypothalamus.
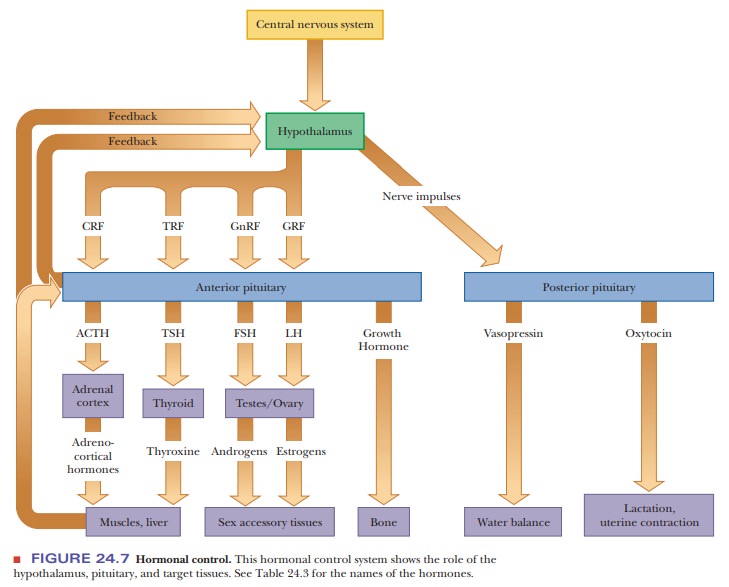
The hypothalamus secretes a hormone-releasing factor, which in turn stimulates release of a
trophic hor-mone by the anterior pituitary (Table 24.3). (The action of the
hypothalamus on the posterior pituitary is mediated by nerve impulses.) Trophic hormones act on specific endocrine glands, which release the
hormones to be transported to target organs. Note that feedback control is
exerted at every stage of the process. Even more fine-tuning is possible with
zymogen activation mechanisms, which exist for many well-known hormones.
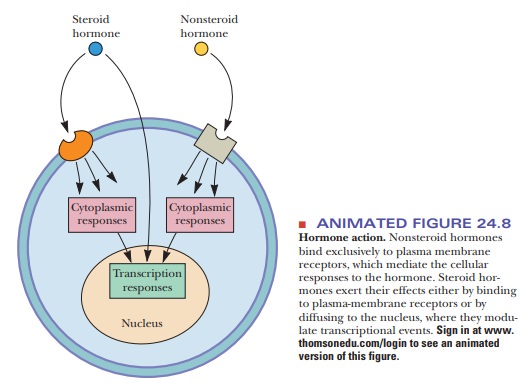
The chemical natures of hormones play a predictably important role
in their roles in cell signaling. Steroid hormones, for example, can enter the
cell directly through the plasma membrane or can bind to plasma membrane
receptors. Nonsteroid hormones enter the cell exclusively as a result of
binding to plasma membrane receptors (Figure 24.8).
The releasing factors and trophic hormones listed in Table 24.3
tend to be polypeptides, but the chemical natures of the hormones released by
specific endocrine glands show greater variation. Thyroxine, for example,
produced by the thyroid, is an iodinated derivative of the amino acid tyrosine.
Abnormally low levels of thyroxine lead to hypothyroidism,
characterized by lethargy and obesity, whereas increased levels produce the
opposite effect (hyperthyroidism).
Low levels of iodine in the diet often lead to hypothyroid-ism and an enlarged
thyroid gland (goiter). This
condition has largely been eliminated by the addition of sodium iodide to
commercial table salt (“iodized” salt). (It is virtually impossible to find
table salt that is not iodized.)
Steroid hormones are produced by the adrenal cortex and the gonads (testes in males, ovaries in females). The adrenocortical hormones include glucocorticoids, which affect carbohydrate metabolism, modulate inflammatoryreactions, and are involved in reactions to stress. The mineralocorticoids control the level of excretion of water and salt by the kidneys. If the adrenal cortex does not function adequately, one result is Addison’s disease, characterized by hypogly-cemia, weakness, and increased susceptibility to stress. This disease is eventually fatal unless it is treated by administration of mineralocorticoids and glucocor-ticoids to make up for what is missing. The opposite condition, hyperfunction ofthe adrenal cortex, is frequently caused by a tumor of the adrenal cortex or of the pituitary. The characteristic clinical manifestation is Cushing’s syndrome, marked by hyperglycemia, water retention, and the easily recognized “moon face.”
The adrenal cortex produces some steroid sex hormones, the androgens and estrogens, but the main site of production is the gonads. Estrogens
are requiredfor female sexual maturation and function, but not for embryonic
sexual devel-opment of female mammals. Animals that are male genetically appear
to be females if they are deprived of androgens during embryonic development.
As a final example, we shall discuss growth hormone (GH), which is a
polypeptide. When overproduction of GH occurs, it is usually because of a
pituitary tumor. If this condition occurs while the skeleton is still growing,
the result is gigantism. If the
skeleton has stopped growing before the onset of GH overproduction, the result
is acromegaly, characterized by
enlarged hands, feet, and facial features. Underproduction of GH leads to dwarfism, but this condition can be
treated by the injection of human GH before the skeleton reaches maturity.
Animal GH is ineffective in treating dwarfism in humans. Supplies of human GH
were very lim-ited when it could be obtained only from cadavers, but it can now
be synthesized by recombinant DNA techniques. Human growth hormone (HGH) has
recently become avail-able to individuals who believe it will help alleviate
the effects of aging. It was known that the level of HGH decreases after middle
age is reached. Many have assumed that the availability of growth hormone, if
one could afford it, would be a virtual fountain of youth. Even though few
results are conclusive at this time, HGH is being prescribed, and the medical
community has adopted rules for its use. For example, doctors will consider
prescribing it only for patients over age
The same hormone is also used illegally by endurance athletes, and
there is currently no reliable test to stop this illegal use.
Second Messengers
When a hormone binds to its specific receptor on a target cell, it
sets off a chain of events in which the actual response within the cell is
elicited. Several kinds of receptors are known. The receptors for steroid
hormones tend to occur within the cell rather than as part of the membrane
(steroids can pass through the plasma membrane); steroid–receptor complexes
affect the transcription of specific genes. More frequently, the receptor proteins
are a part of the plasma membrane. Binding of hormone to the receptor triggers
a change in concentration of a second messenger. The second messenger brings about the changes within the cell as a
result of a series of reactions.
How do second messengers work?
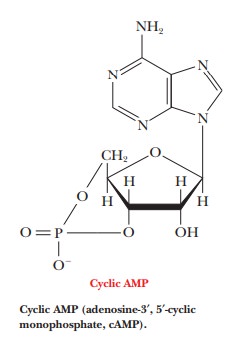
Cyclic AMP and G Proteins
Cyclic
AMP (adenosine-3',5'-monophosphate, cAMP) is one example of a second messenger.
The mode of action starts with binding of a hormone to a specific receptor
called a β1- or β2-adrenergic receptor, which triggers the production
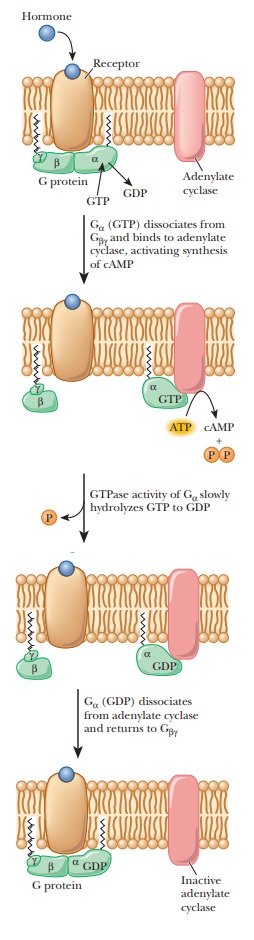

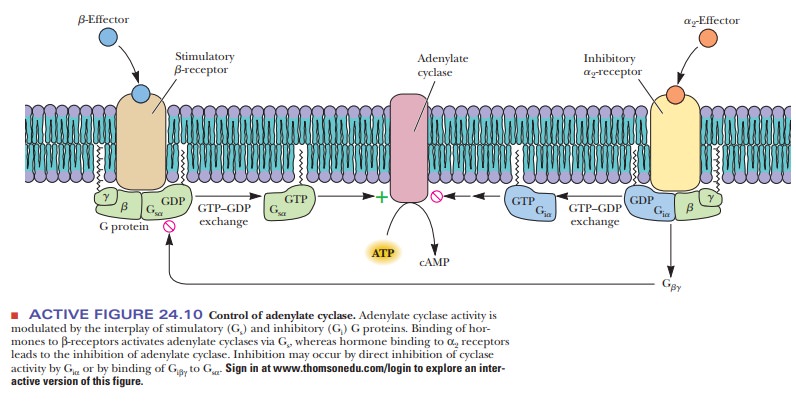
This reaction is mediated by a stimulatory G protein, a trimer consisting of
three subunits—α, β, and γ. Binding of the hormone to the receptor activates
the G protein; the α-subunit binds GTP while releasing GDP, giving
rise to the name of the protein. The active protein has GTPase activity and
slowly hydrolyzes GTP, returning the G protein to the inactive state. GDP
remains bound to the α-subunit and must be exchanged for GTP when the
protein is activated the next time (Figure 24.9). The G protein and adenylate
cyclase are bound to the plasma membrane, while cAMP is released into the
interior of the cell to act as a second messenger. As we have already seen in
several pathways, cAMP stimulates protein kinase A, which phosphorylates a host
of enzymes and transcription factors. Some examples are known in which the
binding of hormone to receptor (anα2-receptor) inhibits rather than stimulates
adenylate cyclase. A G protein with a different kind of α-subunit mediates the process. The modified G protein is referred
to as aninhibitory G protein to
distinguish it from the kind that stimulates response tohormone binding (Figure
24.10).
In
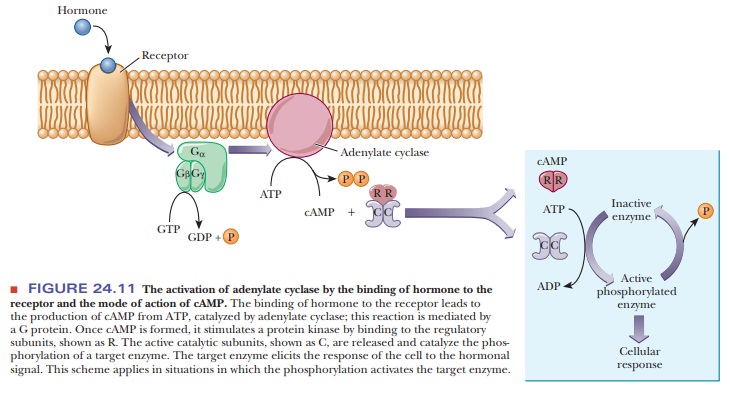
eukaryotic cells, the usual mode of action of cAMP is to stimulate a
cAMP-dependent protein kinase, a tetramer consisting of two regulatory subunits
and two catalytic subunits. When cAMP binds to the dimer of regulatory
subunits, the two active catalytic subunits are released. The active kinase
catalyzes the phosphorylation of a target enzyme or transcription factor
(Figure 24.11). In the scheme shown in Figure 24.11, phosphorylation activates
the enzyme. Cases are also known in which phosphorylation inactivates a target
enzyme. The usual site of phosphorylation is the hydroxyl group of a serine or
a threonine. ATP is the source of the phosphate group that is trans-ferred to
the enzyme. The target enzyme then elicits the cellular response.
G proteins are very important signaling molecules in eukaryotes. They can be activated by combinations of hormones. For example, both epinephrine and glucagon act via a stimulatory G protein in liver cells. The effect can be cumulative, so that if both glucagons and epinephrine have been released, the cellular effect is greater. Besides the effect on cAMP, G proteins are involved in activating many other cellular processes, including stimulating phospholipase C and opening or closing membrane ion channels. They are also involved in vision and smell. There are currently more than 100 known G protein–coupled receptors and more than 20 known G proteins.
A G protein is permanently activated by cholera toxin, leading to excessive
stimulation of adenylate cyclase and chronic elevation of cAMP levels. The main
danger in cholera, caused by the
bacterium Vibrio cholerae, is severe
dehy-dration as a result of diarrhea. The unregulated activity of adenylate
cyclase in epithelial cells leads to the diarrhea because cAMP in epithelial
cells stimulates active transport of Na+.
Excessive cAMP in the epithelial cells of the small intes-tine produces a large
flow of Na+ and water from the mucosal surface of the epithelial cells into the
lumen of the intestine. If the lost fluid and salts can be replaced in cholera
victims, the immune system can eliminate the actual infec-tion within a few
days.
Calcium Ion as a Second Messenger
Calcium
ion (Ca2+) is involved in another ubiquitous second-messenger
scheme. Much of the calcium-mediated response depends on release of Ca2+
from intracellular reservoirs, similar to the release of Ca2+ from
the sarcoplasmic reticulum in the action of the neuromuscular junction. A
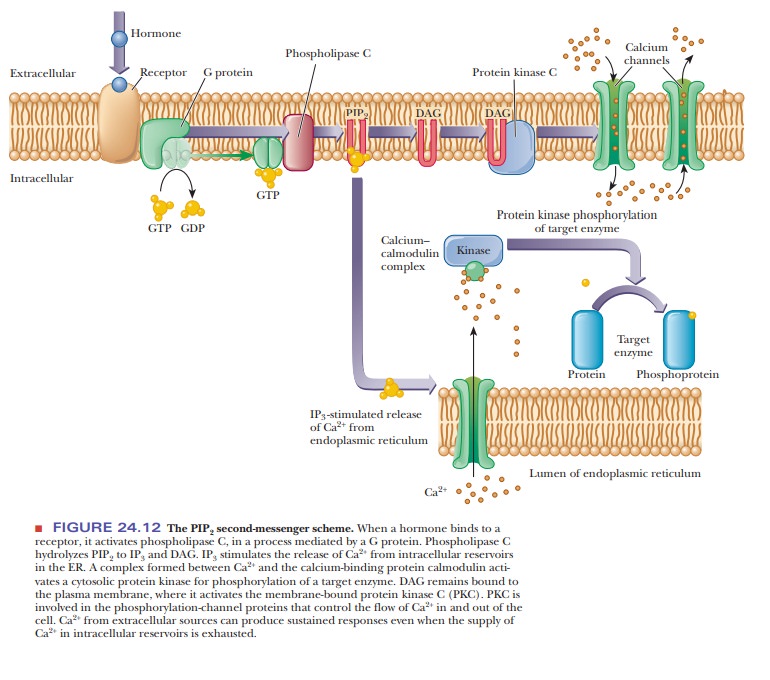
component of the inner layer of the phospholipid bilayer, phosphatidylinositol 4,5-bisphosphate
(PIP2), is also required in this scheme (Figure 24.12).
When the
external trigger binds to its receptor on the cell membrane, it activates phospholipase C, which hydrolyzes PIP2
to inositol 1,4,5-triphosphate (IP3)
and a diacylglycerol (DAG), in a
process mediated by a differentmember of the family of G proteins. The IP3
is the actual second messenger. It diffuses through the cytosol to the
endoplasmic reticulum (ER), where it stimulates the release of Ca2+.
A complex is formed between the calcium-binding protein calmodulin and Ca2+.
This calcium–calmodulin complex activates a cyto-solic protein kinase, which
phosphorylates target enzymes in the same fashion as in the cAMP
second-messenger scheme. DAG also plays a role in this scheme; it is nonpolar
and diffuses through the plasma membrane. When DAG encoun-ters the
membrane-bound protein kinase C, it too acts as a second messenger
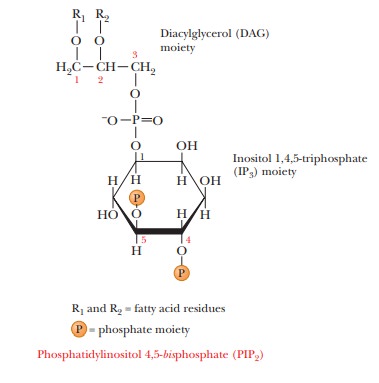
Protein kinase C also phosphorylates target enzymes, including channel proteins that control the flow of Ca2+ into and out of the cell. By controlling the flow of Ca2+, this second-messenger system can produce sustained responses even when the supply of Ca2+ in the intracellular reservoirs becomes exhausted.
Receptor Tyrosine Kinases
Another important type of second-messenger system involves a
receptor type called a receptor tyrosine
kinase. These receptors span the membrane of the cell and have a hormone
receptor on the outside and a tyrosine kinase portion on the inside. There are
several subclasses of these receptor kinases, as shown in Figure 24.13. The
best known of these is class II, which includes the insulin receptor.
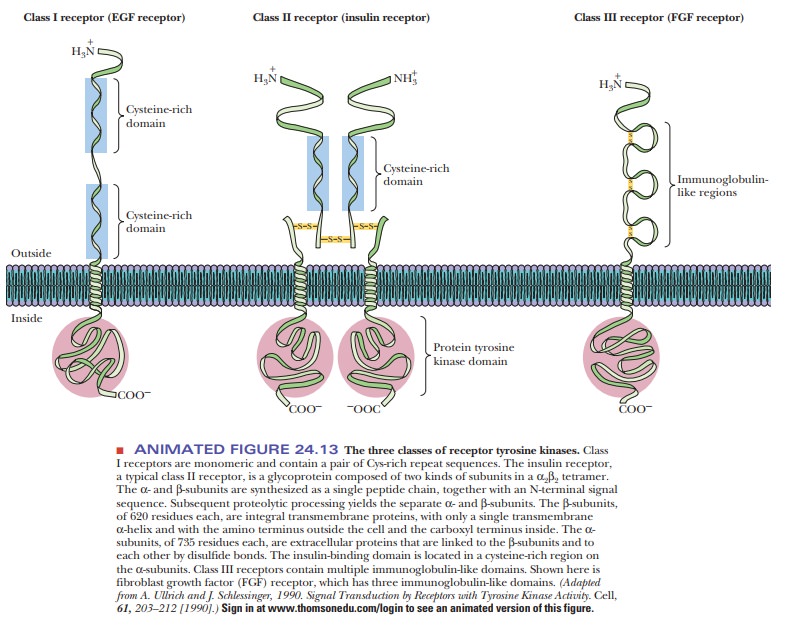
These kinases are allosteric enzymes. When the hormone binds to the bind-ing region on the outside of the cell, it induces a conformational change in the tyrosine kinase domain that activates the kinase activity.
The
activated tyrosine kinases phosphorylate tyrosines on a variety of target
proteins, causing altera-tions in membrane transport of ions and amino acids
and the transcription of certain genes. Phospholipase C (seen in Figure 24.12)
is one of the targets of tyrosine kinases. Another is an insulin-sensitive protein kinase, which phos-phorylates and
activates protein phosphatase 1.
Summary
Sophisticated fine-tuning of metabolic processes in multicellular
organisms is possible through the actions of hormones and second messengers.
In humans, a complex hormonal system has evolved that requires
releas-ing factors (under the control of the hypothalamus), trophic hormones
(under the control of the pituitary), and specific hormones for target organs
(under the control of endocrine glands).
Feedback control occurs at every level of the system.
One important system involves hormones that stimulate a
membrane-bound G protein, which then stimulates adenylate cyclase to produce cAMP.
In these cases, cAMP is the second messenger.
In another important system, a hormone stimulates a different G
protein that then stimulates phospholipase C. Phospholipase C converts
phospha-tidylinositol 4,5-bisphosphate
(PIP2) to diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3), both
of which stimulate the opening of calcium channels and the release of Ca2+. In
this scenario, the Ca2+ is the second messenger.
Receptor tyrosine kinases are a third important type of membrane
protein involved in second-messenger systems.
Related Topics