Chapter: Pharmaceutical Drug Analysis: Gas Liquid Chromatography (GLC)
High Performance Liquid Chromatography (HPLC): Theory
THEORY
The particle size of the stationary phase material plays
a very vital and crucial role in HPLC. In fact,
high-efficiency-stationary-phase materials have been researched and developed
exclusively for HPLC having progressively smaller particle size termed as
microparticulate column packings. These silica particles are mostly uniform,
porous, with spherical or irregular shape, and having diameter ranging from 3.5
to 10 μ m.
Bonded-Phase Supports :
The bonded-phase supports
usually overcome plethora of the nagging prob-lems which is mostly encountered
with adsorbed-liquid phases. Here the molecules, comprising the stationary
phase, i.e., the surfaces of the
silica particles, are covalently bonded to a silica-based support particle.
However, the most popular bonded-phase, siloxanes, are formed by heating the
silica particles-in dilute acid for a day or two so as to generate the reactive
silonal group :
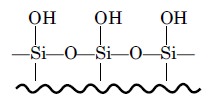
which is subsequently treated with an organochlorosilane
:
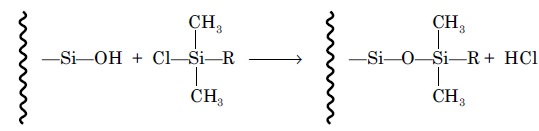
These bonded phases are found to be fairly stable between
the pH range 2 to 9 and upto temperatures of about 80 °C. The nature of the R
group of the silane solely determines the surface polarity of the bonded phase.
A fairly common bonded phase is made with a linear C18 hydrocarbon,
also known as ODS (octadecyl silane) bonded phases, wherein the groups appear
to be protruding out from the silica particle surface just as the bristles on a
toothbrush. It takes care of almost 75% of the samples in HPLC.
Note : The exact mechanism by
which the respective bonded phases actually alter the nature of the sorption
mechanism is still not yet clear.
When such microparticulate-bonded-phases are packed
compactly into a column by means of a suit-able device, the small size of these
particles offers a significant resistance to solvent flow ; therefore, the
mobile phase has to be pumped through the column under a high positive
pressure. For an analytical HPLC, the mobile-phase is pumped through the column
at a flow rate of 1-5 cm3. min–1.
At this juncture usually two varying situations arise.
These are, firstly, isocratic elution
- i.e., when the composition of the
mobile-phase is constant, and
Secondly, gradient elution-i.e., when the composition of the mobile
phase can be made to change in a predetermined
fashion during the course of separation.
Note : Here, the gradient
elution may be simply compared to the temperature programming in GC.
In-line Detector :
It broadly helps to sense the
separated solutes, after they exit through the column. Invariably the detector is an electrical signal whose variation is
displayed on a potentiometer recorder or a computing integrator or a
video-screen. Modern HPLC units are provided with detectors having
selective-devices thereby categorically restricting the response to all the
solutes present in a mixture.
Note : However, no universal
detector has so far been discovered for HPLC to cater for a wide-spectrum of
components ; as the Flame-Ionization-Detector (FID) used for GC.
Post-Column Derivatisation :
There are certain stubborn and
fairly difficult components that are not
easily detectable in HPLC. Therefore, such component(s) have to be
appropriately converted into their corre-sponding detectable form once they
emerge from the column.
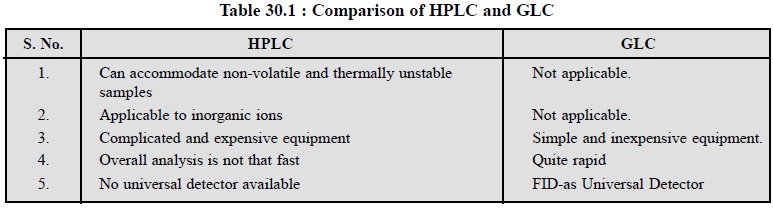
Table 30.1 records the comparison of HPLC and GLC
specifically with respect to their advantages.
Both HPLC and GLC are :
·
Efficient, highly selective and widely applicable
·
Only small quantum of sample required
·
Ordinarily non-destructive of sample
·
Readily adaptable to ‘Quantitative
Analyses’
·
Provide dependable, accurate and precise and reproducible
results.
Related Topics