Chapter: Pharmaceutical Drug Analysis: Gas Liquid Chromatography (GLC)
Applications of GLC in Pharmaceutical Analysis
APPLICATIONS OF GLC IN PHARMACEUTICAL ANALYSIS
Gas liquid chromatography (GLC) or gas chromatography (GC) finds its abundant applications in the accurate and precise analysis of
plethora of official pharmaceutical substances covering a wide range as
enumerated below :
(i) Assay of
Drugs,
(ii)
Determination of specific organic compounds as impurities in official
pharmaceutical substance,
(iii)
Determination of related substances in official drugs,
(iv)
Determination of water in drug, and
(v)
Determination of chloroform with head-space chromatography.
1. ASSAY OF DRUGS
Assay of Cetostearyl Alcohol
Materials Required : Solution (1) (1% w/v of
cetostearyl alcohol sample in 96% ethanol) ; solution (2) (0.6% w/v of cetyl
alcohol EPCRS in 96% ethanol) ; (3) (0.4% w/v of stearyl alcohol EPCRS in 96%
ethanol ; solution (4) [mix 1 ml of solution (2) and 1 ml of solution (3) and
add sufficient 96% ethanol to produce 10 ml] ;
Chromatographic Parameters-are as follows :
(i) Column : Made of glass or stainless
steel ; size : (3 M × 4 mm) ; adsorbent : diatomaceous support (125 to 180
mesh) impregnated with 10% w/w of polydimethylsiloxane and maintained at 200
°C,
(ii) Inlet-port and Detector : are
maintained at 250 °C,
(iii) Flow rate of Carrier Gas (N2)
: 30 ml minute–1, and
(iv) Resolution Factor : between the two
principle peaks in the chromatogram obtained with solution (1) must not be less
than 1.25 (it may be achieved by adjusting the flow rate), and
(v) Detector : Flame Ionization Detector
(FID).
Procedure : After having maintained the
above mentioned experimental conditions for gas chromatog-raphy inject 2 μ l of solutions (1) through (4)
sequentially.
Observations : The assay is not valid unless
the chromatogram obtained with solution (4) shows two principal peaks with a signal-to-noise ratio of at least 5.
Calculations : Calculate the content of
cetylalcohol and of stearyl alcohol from the chromatogram thus obtained with solution (1) by
normalization. Identify the peaks by visual comparison with the chromatograms
obtained with solutions (2) and (3) respectively.
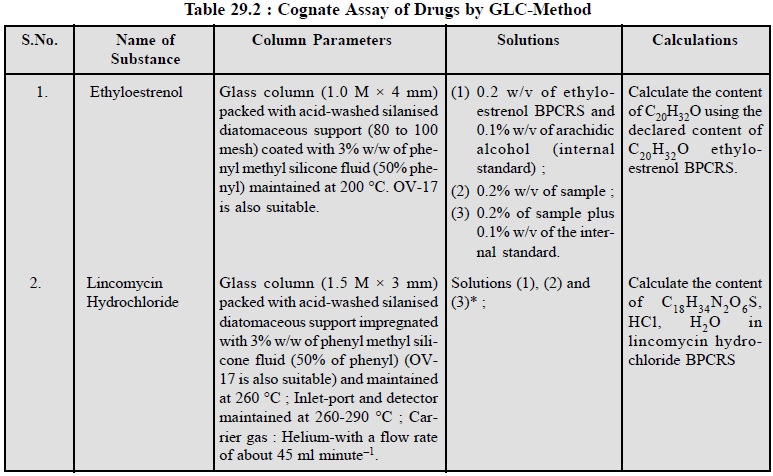
1.1. Cognate Assays
A few other drugs can also be assayed by the same
procedure and are stated below in Table 29.2 :
2. DETERMINATION OF SPECIFIC ORGANIC COMPOUNDS AS IMPURITIES IN OFFICIAL PHARMACEUTICAL
SUBSTANCES
A number of organic compounds, such as : N,
N-dimethylaniline-present in amoxycillin trihydrate ; cephalexin ; cloxacillin
sodium ; dicloxacillin sodium ; 2-ethylhexanoic acid-in amoxycillin sodium ;
4-chlorophenol-in clofibrate ; acetone and butanol-in daunorubacin
hydrochloride ; cineole : limonene ratio-in dementholised mint oil etc ;
A. Determination of N,
N-dimethylaniline in Cephalexin
Materials Required : Cephalexin sample : 1.0 g ;
Solution A [0.005% w/v soln. of naphthalene (inter-nal standard) in
cyclohexane] : 20 ml ; Solution B (mix 50 mg of N, N-dimethylaniline with 2 ml
HCl and 20 ml of water, shake to dissolve, add enough DW to produce 50 ml ; and
dilute 5 ml of the resulting solution to 250 ml with DW) : 20 ml ; solution (1)
(add 5 ml of 1 M NaOH and 1 ml of solution A to 1 ml of solution B, shake
vigorously for 1 minute, centrifuge and use the upper layer) ; solution (2)
(dissolve 1 g of cephalexin sample in 5 ml of 1 M NaOH, add 1 ml of solution A,
shake vigorously for 1 minute, centrifuge and use the upper layer) ;
Chromatographic Conditions : are as stated under :
Column* : Glass column ; size (2 M × 2 mm) ; adsorbent : packed with
acid-washed, silanized diatomaceous support (80 to 100 mesh) impregnated with
3% w/w of phenyl methyl sili-cone fluid (50% phenyl, and maintained at 120 °C,
Detector : Flame Ionization Detector
(FID),
Inlet-port and Detector : maintained at 150 °C, and
Flow Rate : 30 ml minute–1 for N2 as the carrier gas.
Procedure : After having set the above
experimental conditions for gas chromatography, inject 1 μ l of the
solutions (1) and (2) sequentially into the column. Repeat the determinations
so as the ensure a consistent response. Determine the peak areas**.
Calculations : From the value obtained
calculate the content of N, N-dimethylaniline present in the given sample of cephalexin. However,
according to BP(1993) it should not be more than 20 ppm.
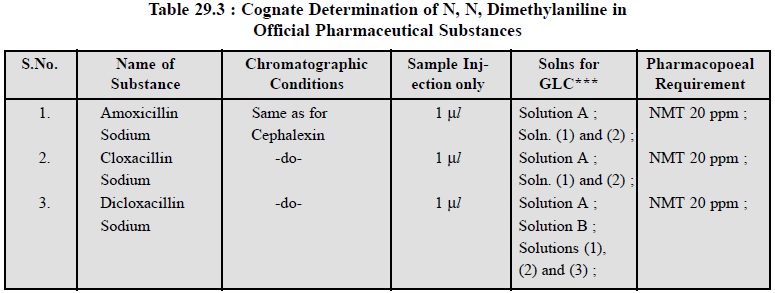
2.1. Cognate Assay
A few cognate determinations are listed in Table 29.3 :
3. DETERMINATION OF RELATED SUBSTANCES IN OFFICIAL DRUGS
Related substance present in good number of official
drugs may be determined by GLC method, for instance : Bromopheneramine maleate
; Bronopol ; Cepahloridine ; Chlorocresol ; Chloroform ; Chloroxylenol.,
Cindamycin hydrochloride ; Griseofulvin ; Isometheptene mucate ; Levomenthol ;
A. Related Substances in
Bromopheneramine Maleate
Materials Required : Solution (1) dilute 1 volume
of solution (3) to 200 volumes with a 0.005% w/v solution of N-phenylcarbazole (internal standard) in toluence : 10
ml ; Solution (2) : (add 5 ml of DW to 0.1 g of bromopheneramine maleate
sample, make the resulting solution alkaline with 13.5 M ammonia, add 2.5 ml of
toluene, shake for 5 minutes, centrifuge and use the upper layer) ; solution
(3) : (prepare it exactly in the same manner as solution-‘2’ but using the
internal standard solution in place of toluene) ; Solution (4) : (dis-solve 10
mg of bromopheneramine maleate BPCRS in 5 ml of DW, make alkaline with 13.5 M
ammonia, add 2.5 ml of toluene, shake for 5 minutes, centrifuge and dilute 1
volume of the upper layer to 20 volumes with toluene) ;
Chromatographic Parameters : These are as mentioned below :
Column : Glass column ; size : (1.5 m ×
4 mm) ; adsorbent : packed with acid-washed, silanized diatomaceous support ( 80 to 100 mesh) impregnated with 3% of
phenyl methyl silicone fluid (50% phenyl) and maintained at 220 °C.
Flow rate of Carrier Gas : 30 l minute–1 ;
Carrier Gas : Nitrogen ;
Detector : Flame Ionization Detector
(FID) ;
Procedure : After having maintained the
aforesaid experimental parameter for gas chromatography, inject 1 µl each of solutions (1), (2),
(3) and (4) in a sequential manner.
Calculations
(i) Calculate
the ratio (γ) of the area of the peak due to bromopheneramine to that
of the peak due to the internal standard in the chromatogram obtained with
solution (1) ; and
(ii) In the
chromatogram obtained with solution (3) the ratio of the sum of the areas of
any secondary peaks to the area of the peak due to the internal standard is not
greater than γ and the ratio of the area of any secondary peak to the
area of the peak due to the internal standard is not greater than 0.4γ.
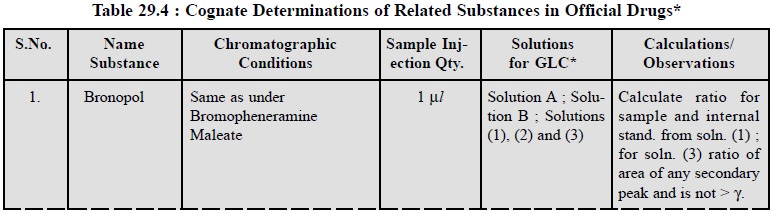
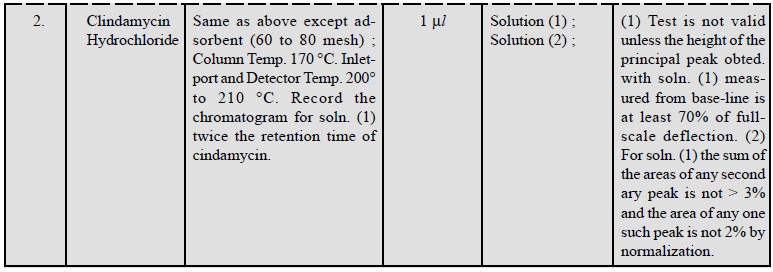
3.1. Cognate Determinations
A few cognate assays are summarized in Table 29.4 ; below
:
4. Determination of Water in a Drug
Assay of Water Present in
Mentrophin
Materials Required : Solution (1) [dilute 15 μ l of anhydrous methanol (internal standard) with
suffi-cient anhydrous propan-2-ol to produce 100 ml] ; Solution (2) (dissolve 4
mg of mentrophin sample in 0.5 ml of anhydrous propan-2-ol) ; Solution (3)
[dissolve 4 mg of mentrophin sample in 0.5 ml of solution (1)] ; Solution (4)
and 10 μ l of water to 50 ml of solution
(1) ;
Chromatographic Conditions
Column : Stainless steel ; size : (1 M
× 2 mm) ; adsorbent : packed with porous polymer beads (60 to 80 mesh) and maintained at 120 °C.
[Note :
Chromosorb 102 is also suitable.]
Carrier Gas : Helium ;
Detector : Thermal Conductivity Detector
(TCD)-maintained at 150 °C.
Procedure : After having maintained the
various experimental parameters stated above for gas chro-matography and using
throughout absolutely dry glassware which may be siliconized, inject 1 μ l of solution (1) through
solution 4 sequentially and obtain the chromatogram.
Calculations : From the chromatograms
obtained and taking into account any water detectable in solution (1), calculate the percentage of water taking 0.9972 g as
its weight per ml at 20 °C.
5. DETERMINATION OF CHLOROFORM IN COLCHICINE BY HEAD-SPACE GAS CHRO-MATOGRAPHY
Head-space gas chromatography is an analytical device
specifically suitable for the separation and simultaneous determination of
volatile constituents present in solid or in liquid samples.
Principle : The underlying principle of head space gas chromatography is the
analysis of the vapour phase in
equilibrium with the solid or liquid phase.
Apparatus : The introduction of sample(s)
may be accomplished by using airtight syringes and a simple conventional gas chromatograph. Nevertheless, the
equilibrium has got to be carried out in a separate chamber and the vapour
phase is subsequently conveyed to the column taking necessary and every
possible precautions so as to avoid any minute changes in the equilibrium.
Materials Required : Solution (1) dissolve 0.4 g of
colchicine sample in sufficient water to produce 10 ml and place 1 ml of the solution in each of three identical
stoppered vials ; solution (2) dilute 5 μ l of chloro-form to 10 ml with
water and place 10 μ l of the resulting solution and
1 ml of solution (1) in each of three identical stoppered vials ;
Chromatographic Conditions
Column : Fused-silica capillary column
; size : (50 M × 0.32 mm) coated with a 5- μ m film of chemi-cally-bonded
polymethyl siloxane ;
Detector : Flame Ionization Detector
(FID) maintained at 250 °C.
Carrier Gas : Nitrogen
Flow Rate : 4 ml minute–1 for the carrier gas ;
Procedure : First of all maintain the
above experimental parameters of the gas chromatograph and then maintain the six solutions at 90 °C
for 20 minutes, pressurise for a duration of 30 seconds only and transfer
subsequently to the column at a temperature of 120 °C. Repeat the operation
using a vial containing 1 ml of water. Perform each measurement at least three
times.
Calculations : Calculate the percentage w/w
of chloroform, taking into consideration 1.48 as the weight per ml at 20 °C.
Related Topics