Chapter: Pharmaceutical Drug Analysis: Gas Liquid Chromatography (GLC)
Applications of High Performance Liquid Chromatography (HPLC) in Pharmaceutical Analysis
APPLICATIONS OF HPLC IN PHARMACEUTICAL ANALYSIS
Modern HPLC finds its abundant applications not only
confined to isolation of natural pharmaceutically active compounds, control of
microbiological processes but also assay of pure drugs and their dosage forms.
A few typical examples will be discussed below :
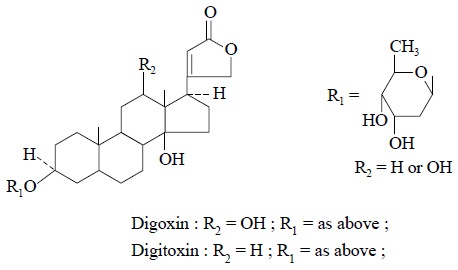
1. ISOLATION OF NATURAL PHARMACEUTICALLY ACTIVE COMPOUNDS
Some plant alkaloids and glycosides can be isolated as
stated below :

Chromatographic Conditions :
Column : Size-25 cm ×
4.6 mm ID ;
Adsorbent : Lichrosorb RP-8 ;
Mobile-phase : Water/Acetonitrile-Gradient
Elution ;
Detector : UV 254 nm
2. CONTROL OF MICROBIOLOGICAL PROCESSES
Various microbiological processes are used in the
production of a number of antibiotics, for instance :
penicillins, tetracyclines,
chloramphenicol and streptomycins.
The major areas of such operations being :
·
kinetics of the microbiological process,
·
monitoring of the on-going process,
·
isolation and purification of active ingredients,
·
purity control of active constituents, and
·
monitoring derivatization reactions of these compounds.
HPLC-controlled analysis of a
microbiological process during Penicillin Production : Chromatographic conditions are as follows :
Column : Size-25 cm × 4.6
mm ID ;
Adsorbent : Lichrosorb-NH2(R)
(10 μ m) ;
Mobile-phase : 0.005 M H2SO4 buffer (pH 4.4))/acetonitrile (50 :
50) ; Flow rate : 3 ml min–1 ;
Detector : UV-220 nm ;
Microbial cleavage of Penicillin-G into 6-AMP and
phenylacetate is as shown below :
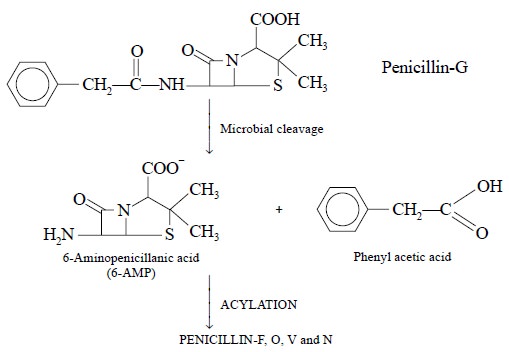
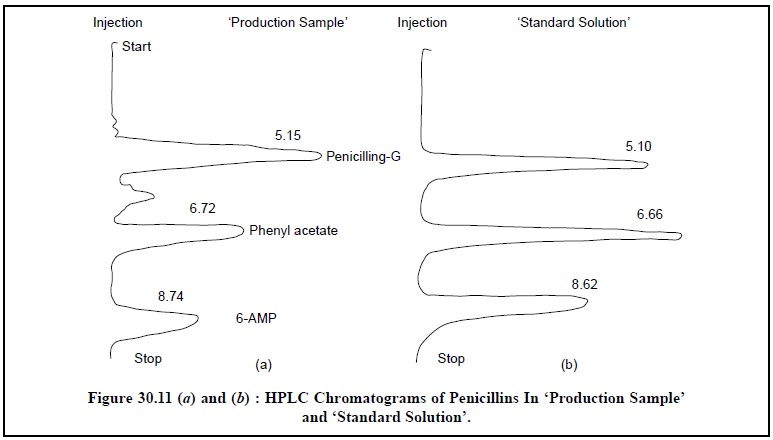
Figure : 11 (a)
and (b) shows the HPLC chromatograms
of penicillins in ‘production sample’
and ‘standard solution’ respectively
:
3. ASSAY OF CEPHALOSPORINS
Several commercially available cephalosporin antibiotics
have been adequately separated by HPLC methods under the following experimental
parameters
Column : ODS-SIL-X-II,
Mobile-phase : 0.95 M Ammonium
Carbonate/Methanol (95 : 5) ;
Detector :
UV-220 nm ;

The loss of absorption with cleavage of the β-lactam was used by Marrelli*
to analyze the concentra-tions of cephalosporin C in the presence of other UV
absorbing species.
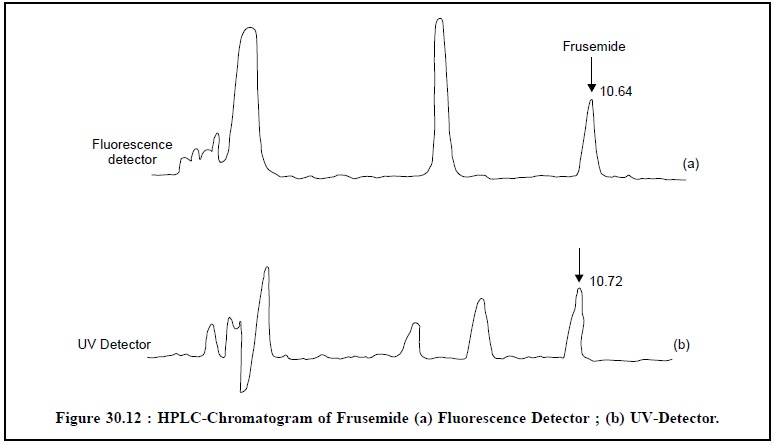
4. ASSAY OF FRUSEMIDE**
Theory : HPLC analysis of frusemide and
its decomposition products is carried out by using simultane-ous fluorescence
and UV detection.
It is noteworthy that fluorescence detection is a very
specific technique, especially when excitation and emission wavelengths can be
selected. In addition to this, sensitivity for compounds with photoluminescence
properties can be higher by factors of 100 to 1000 when compared with that of
other detectors.
The chromatographic conditions for Frusemide
determination are as stated below :
Column : Size : 250 × 4.6 mm ID ;
Adsorbent : Lichrosorb(R) RP-8, 10 μ m ;
Mobile-phase : Gradient elution-2 minutes, from 20% B to 37% B in 15
minutes, where, A = Water (pH 2.7) and B = Acetonitrile ;
Detector : (i)
Fluorescence : Excitation : 275 nm ; Emission : above 405 nm ;
Figure : 12 (a)
and (b) depict the HPLC chromatograms
of frusemide by fluorescence and UV detectors respectively.
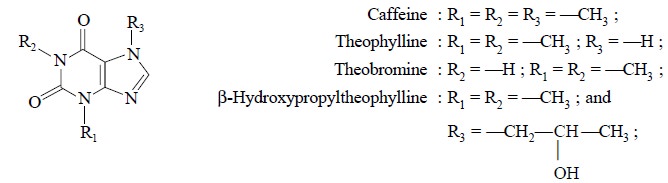
5. ASSAY OF THEOPHYLLINE
Theophylline invariably contains other related substances
as impurities, namely : theobromine, caffeine and β-hydroxypropyltheophylline.
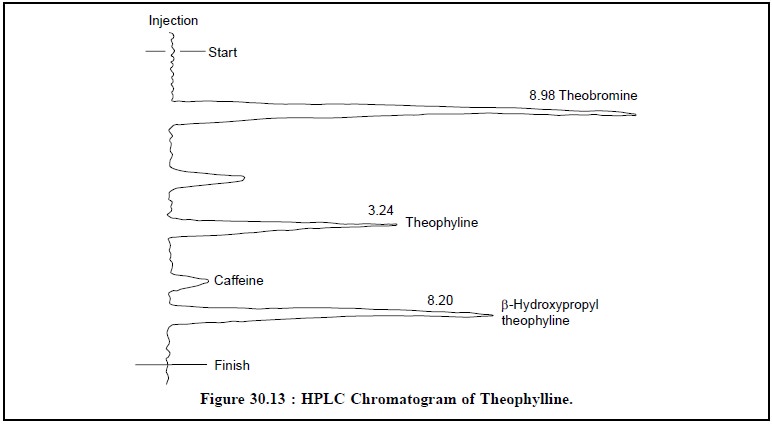
The chromatographic
conditions for HPLC are as stated below :
Sample size : 10
µL ;
Column :
size – 250 × 4.6 mm ID ;
Adsorbent :
Lichrosorb (R) RP-8, 10 µm ;
Mobile-phase :
0.02 M KH2PO4 Buffer (pH 3.5)/Acetonitrile (95 : 5) ;
Detector : UV-254
nm ;
Figure 30.13, illustrates the HPLC chromatogram of theophylline assay with four distinct peaks.
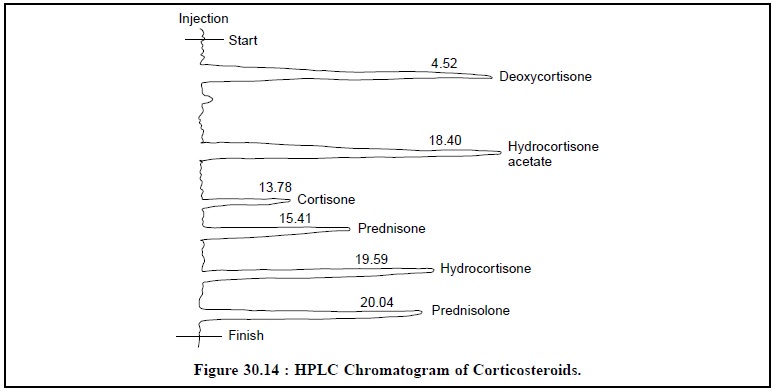
6. ASSAY OF CORTICOSTEROIDS
mixture of six
corticosteroids, namely : deoxycortisone,
hydrocortisone acetate, cortisone, prednisone, hydrocortisone and prednisolone can be assayed by HPLC
method. The chromatogrpahic param-eters for the assay are as follows :
Sample size : 10 μ L
Column : size-250 × 4.6
mm ID ;
Adsorbent : Lichrosorb(R)
DIOL : 10 μ m ;
Mobile-phase : Gradient elution of A (n-Hexane) and B (Isopropanol) ;
Detector : UV-254 nm ;
Figure 30.14, clearly shows the six well-elaborated and
distinct peaks of all the constituents stated earlier under the above HPLC
parameters.
7. ASSAY OF DICHLORPHENAMIDE*
Materials Required : Dichlorphenamide sample : 100
mg ; dichlorphenamide RS : 100 mg ; Mobile-phase [solution containing 0.02 M
NaH2PO4 and 0.2 M Na2HPO4 in a
mixture of equal volumes of acetonitrile and water] : 50 ml ;
Procedure : The chromatographic procedure
may be performed using μ Bondapack C18 column as the stationary phase and the above mentioned mobile-phase with a
flow rate of 1.0 ml per minute and a detection wavelength of 280 nm. Carry out
the HPLC analysis using solutions in the mobile-phase containing (1) 0.05% w/v
of dichlorphenamide RS and (2) 0.05% w/v of dichlorphenamide sample.
Calculations : Calculate the content of C6H6Cl2N2O4S2 using the declared content of the same
in dichlorphenamide RS.
8. ASSAY OF HUMAN INSULIN*
Materials Required : Solution (1) : dissolve 40 mg
of Human Insulin in sufficient of 0.01 M HCl (0.3648 g of HCl in one litre DW) to produce 10 ml ; solution (2)
: Mix thoroughly 900 μ L of 0.01 M HCl to 100 μ L of solution (1) ; Solution (3) : dissolve 40 mg of
Human Insulin EPCRS in sufficient 0.01 M HCl to produce 10 ml ; Solution (4) :
mix 1 ml of solution (3) with 1 ml of a solution containing 4 mg of porcine
insulin RS : Mobile-phase ‘A’ : Dissolve 28.4 g of anhydrous Na2SO4
in sufficient water to produce 1000 ml, add 2.7 ml of orthophosphoric acid,
adjust of pH 2.3, if necessary, with ethanolamine, filter and degas by passing
He through the solution ; Mobile-phase ‘B’ : Mix 500 ml of mobile-phase ‘A’
with 500 ml of acetonitrile, filter and degas by passing He through the
solution.
Procedure : The HPLC is carried out using
(a) a Vydac C18 column, for proteins and peptides, maintained at 40 °C, (b)
as the mobile phase at a flow rate of 1 ml per minute, a mixture of 48 volumes
of mobile phase ‘A’ and 52 volumes of mobile phase ‘B’ prepared and maintained
at a temperature of not less than 20 °C, and (c) a detection wavelength of 214 nm.
Adopt the following steps sequentially :
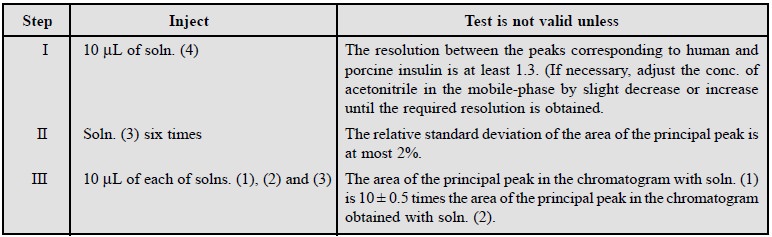
Calculations : Calculate the content of human
insulin, C257H383N65O77S6,
from the peak areas and using the
declared content of C257H383N65O77S6
in human insulin EPCRS.
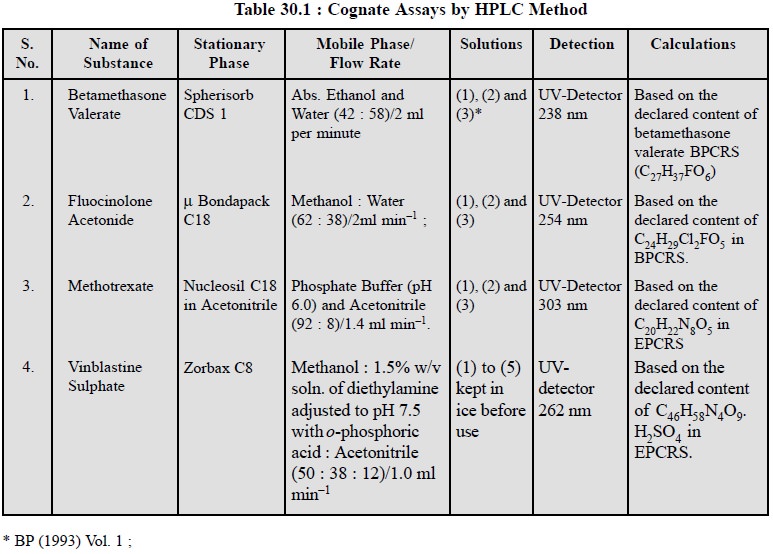
9. COGNATE ASSAYS
A number of pharmaceutical substances can be assayed by
HPLC method as detailed in Table 30.1.
Related Topics