Chapter: Environmental Biotechnology: Microbes and Metabolism
Glycolysis: TCA and Glyoxalate cycle
Glycolysis
As the name implies, glycolysis is a process describing the splitting of a phosphate derivative of glucose, a sugar containing six carbon atoms, eventually to produce two pyruvate molecules, each having three carbon atoms. There are at least four pathways involved in the catabolism of glucose. These are the Embden – Meyerhof (Figure 2.1), which is the one most typically associated with glycolysis, the Ent-ner – Doudoroff and the phosphoketolase pathways and the pentose phosphate cycle, which allows rearrangement into sugars containing 3, 4, 5, 6 or 7 carbon atoms. The pathways differ from each other in some of the reactions in the first half up to the point of lysis to two three-carbon molecules, after which point the remainder of the pathways are identical. These routes are characterised by the particular enzymes present in the first half of these pathways catalysing the steps between glucose and the production of dihydroxyacetone phosphate in equilib-rium with glyceraldehyde 3-phosphate. All these pathways have the capacity to produce ATP and so function in the production of cellular energy. The need for four different routes for glucose catabolism, therefore, lies in the necessity for the supply of different carbon skeletons for anabolic processes and also for the provision of points of entry to glycolysis for catabolites from the vast array of functioning catabolic pathways. Not all of these pathways operate in all organ-isms. Even when several are encoded in the DNA, exactly which of these are active in an organism at any time, depends on its current metabolic demands and the prevailing conditions in which the microbe is living.
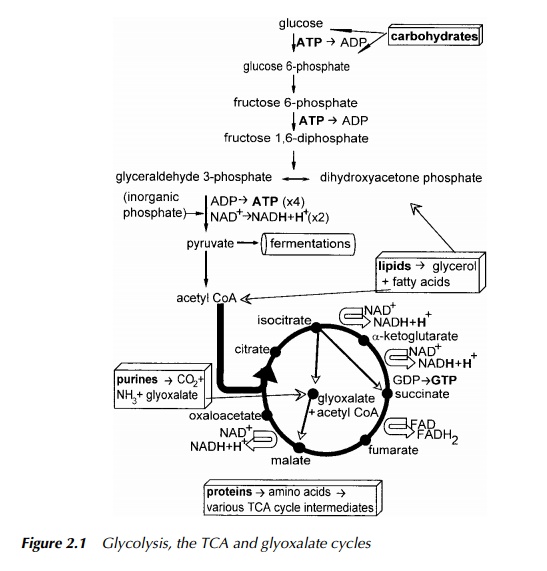
The point of convergence of all four pathways is at the triose phosphates which is the point where glycerol as glycerol phosphate enters glycolysis and so marks the link between catabolism of simple lipids and the central metabolic pathways. The addition of glycerol to the pool of trioses is compensated for by the action of triose phosphate isomerase maintaining the equilibrium between glyceraldehyde 3-phosphate and dihydroxyacetone phosphate which normally lies far in favour of the latter. This is perhaps surprising since it is glyceraldehyde 3-phosphate which is the precursor for the subsequent step. The next stage is the introduction of a second phosphate group to glyceraldehyde 3-phosphate with an accompanying oxidation, to produce glyceraldehyde 1,3-diphosphate. The oxidation involves the transfer of hydrogen to the coenzyme, NAD, to produce its reduced form, NADH. In order for glycolysis to continue operating, it is essential for the cell or organism to regenerate the NAD+ which is achieved either by transfer of the hydrogens to the cytochromes of an electron transport chain whose operation is associated with the synthesis of ATP, or to an organic molecule such as pyruvate in which case the opportunity to synthesise ATP is lost. This latter method is the first step of many different fermentation routes. These occur when operation of electron transport chains is not possible and so become the only route for the essential regeneration of NAD+. Looking at the Embden – Meyerhof pathway, this is also the third stage at which a phosphorylation has occurred. In this case, the phosphate was derived from an inorganic source, in a reaction which conserves the energy of oxidation.
The next step in glycolysis is to transfer the new phosphate group to ADP, thus producing ATP and 3-phosphoglycerate, which is therefore the first substrate level site of ATP synthesis. After rearrangement to 2-phosphoglycerate and dehydration to phosphoenolpyruvic acid, the second phosphate is removed to produce pyruvic acid and ATP, and so is the second site of substrate level ATP synthesis. As mentioned above, depending on the activity of the electron transport chains and the energy requirements of the cell balanced against the need for certain metabolic intermediates, pyruvate, or its derivatives may now be reduced by accepting the hydrogen from NADH and so continue on a fermentation route or it may be decarboxylated to an acetyl group and enter the TCA cycle. The overall energy balance of glycolysis is discussed later when considering chemical cellular energy production in more detail.
TCA cycle
Pyruvate decarboxylation produces the acetyl group bound to Coenzyme A, ready to enter the TCA cycle otherwise named Kreb’s citric acid cycle in tribute to the scientist who discovered it. Not only is this cycle a source of reduced cofactors which ‘fuel’ electron transport and thus, the synthesis of ATP, but it is also a great meeting point of metabolic pathways. Cycle intermediates are constantly being removed or replenished. During anaerobic fermentation, many of the reactions seen in the TCA cycle are in operation even though they are not linked to electron transport.
Glyoxalate cycle
This is principally the TCA cycle, with two additional steps forming a ‘short circuit’, involving the formation of glyoxalate from isocitrate. The second reaction requires the addition of acetyl CoA to glyoxalate to produce malic acid and thus rejoin the TCA cycle. The purpose of this shunt is to permit the organism to use acetyl CoA, which is the major breakdown product of fatty acids, as its sole carbon source.
Related Topics