Chapter: Environmental Biotechnology: Microbes and Metabolism
Fermentation and respiration
Fermentation and respiration
The electrons derived from the catabolism of the carbon source are eventually either donated to an organic molecule in which case the process is described as fermentation, or donated to an inorganic acceptor by transfer along an electron chain. This latter process is respiration and may be aerobic where the terminal electron acceptor is oxygen, or anaerobic where the terminal electron acceptor is other than oxygen such as nitrate, sulphate, carbon dioxide, sulphur or ferric ion. Unfortunately, respiration is a term which has more than one definition. It may also be used to describe a subset of the respiration processes mentioned above to include only oxidation of organic material and where the ultimate elec-tron acceptor is molecular oxygen. This latter definition is the basis of biological oxygen demand (BOD), which is often used to characterise potential environmen-tal pollutants, especially effluents, being a measure of the biodegradable material available for oxidation by microbes.
Fermentations
In modern parlance, there are many definitions of the term ‘fermentation’. They range from the broadest and somewhat archaic to mean any large-scale culture of micro-organisms, to the very specific, meaning growth on an organic substance and which is wholly dependent on substrate-level phosphorylation. This is the synthesis of ATP by transfer of a phosphate group directly from a high energy compound and not involving an electron transport chain. Additionally, and a source of great confusion, is that fermentation may refer simply to any microbial growth in the absence of oxygen but equally may be used generally to mean microbial growth such as food spoilage where the presence or absence of oxygen is unspecified. The definition used throughout this book, except with reference to eutrophic fermentation discussed, is that of growth dependent on substrate-level phosphorylation.
There are very many fermentation routes but all share two requirements, the first being the regeneration of NAD+ from NADH produced during glycolysis which is essential to maintain the overall reduction: oxidation equilibrium, and the second being that pyruvate, or a derivative thereof, is the electron accep-tor during the reoxidation of NADH. What this means is that all fermentation routes start with pyruvate, the end-point of glycolysis, and proceed along a vari-ety of pathways to an end product indicative, if not diagnostic, of the organism.Fermentation is therefore an option under conditions where there is an active elec-tron transport chain as discussed in the following section, but becomes essential when fermentation is the only method for regenerating NAD+.
As noted above, the end product of fermentation for any given carbon source may be diagnostic of the identity of a specific organism. This is more relevant for bacteria than for yeast or other eukaryotic cells and arises from the predisposition of that organism, to use a particular fermentation pathway. These are described in detail in Mandelstam and McQuillen (1973) and are summarised in Figure 2.8. Identification by the product of carbohydrate catabolism is somewhat specialised and is very thoroughly set out in Cowan and Steel’s Manual for the Identificationof Medical Bacteria (Barrow and Feltham 1993).
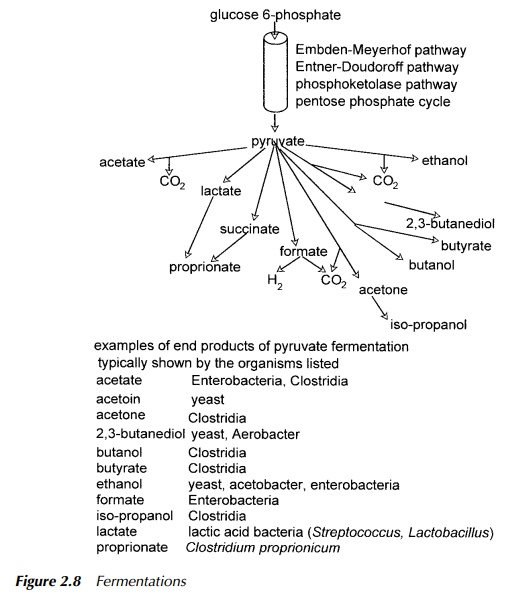
As described in the previous section, NADH and other reduced cofactors may be reoxidised by the reduction of organic receptors such as pyruvate. This is the fermentation route.
Alternatively, the reducing agent (or reductant) can transfer the electrons to an electron transport chain which ultimately donates them to an inorganic receptor (the oxidising agent or oxidant). In aerobic respiration, this receptor is oxygen. However, some bacteria have electron transport chains which use other electron sinks such as nitrate, sulphate, carbon dioxide and some metals, with respiration being described as anaerobic in these cases. The use of nitrate in this role leads to the process of denitrification, which plays an important part in many aspects of the applications of environmental biotechnology.
A number of events occur during the flow of electrons along the chain which have been observed and clearly described for a number of organisms and organelles, most especially the mitochondria of eukaryotic cells. These are fully discussed in many biochemistry textbooks, an excellent example being Lehninger (1975), the gist of which is outlined in this section. The details of exactly how these phenomena combine to drive the synthesis of ATP is still unclear but various models have been proposed.
The chemiosmotic model, proposed by Peter Mitchell in 1961, states that the proton, or hydrogen ion, gradient which develops across an intact membrane during biological oxidations is the energy store for the subsequent synthesis of ATP. This model somewhat revolutionised the then current thinking on the energy source for many cellular processes, as the principles of energy storage and availability according to the chemiosmotic theory were applicable to many energy-demanding cellular phenomena including photosynthetic phosphorylation and some cross-membrane transport systems. It could even account for the move-ment of flagellae which propel those bacteria possessing them, through a liquid medium. The chemiosmotic theory accounts for the coupling of the transmem-brane proton gradient to ATP synthesis. It implies that during oxidation, the electrons flow down from high to low energy using that energy to drive protons across a membrane against a high concentration, thus developing the proton gra-dient. When the electron flow stops, the protons migrate down the concentration gradient, simultaneously releasing energy to drive the synthesis of ATP through membrane-associated proteins. The model system described first is that of mito-chondria and, later, comparisons with bacterial systems associated with oxidative phosphorylation and those systems associated with methanogenesis will be made.
Electron transport chains comprise cytochrome molecules which trap electrons, and enzymes which transfer electrons from a cytochrome to its neighbour. The quantity of energy released during this transfer is sufficient to drive the synthesis of approximately one ATP molecule by the enzyme ATP synthetase. The whole system is located in a membrane which is an essential requirement of any electron transport chain because of the need to organise it topographically, and to allow the establishment of a pH gradient. Also there is evidence that during active electron transport, the morphology of the membrane changes and is believed to store energy in some way yet to be elucidated. Consequently, an intact membrane is essential. Any toxic substance which damages the integrity of a membrane has the potential to interrupt the functioning of the electron transport chain thereby reducing the facility for ATP synthesis and potentially killing the organism. The chain may also be disrupted by interference with the electron carriers. Such a chemical is cyanide, which complexes with cytochrome oxidase, and for which research into a biological remediation route is underway.
The mitochondrial electron transport system and oxidative phosphorylation
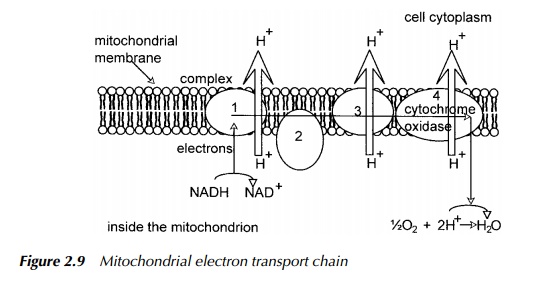
The electron transport system in eukaryotes is located in the inner membrane of mitochondria. A representation of the system is given in Figure 2.9. The chain is a series of complexes comprising cytochromes, and enzymes involved in oxida-tion – reduction reactions whose function is to transfer electrons from one complex to the next. The ratios of the complexes one to another varies from cell type to cell type. However, the concentration of the cytochrome a complex per unit area of inner membrane stays fairly constant. What changes from cell type to cell type is the degree of infolding of the inner membrane, such that cells requiring a large amount of energy have mitochondria which have a very large surface area of inner membrane, which is highly convoluted thus providing a high capacity for electron transport. The process which couples ATP synthesis to electron trans-port in mitochondria and which still evades a complete description, is oxidative phosphorylation or more accurately, respiratory-chain phosphorylation. There are three sites within the mitochondrial chain which span the interaction between two neighbouring complexes, which on the basis of energy calculations are thought to witness a release of energy sufficient to synthesise almost one molecule of
These are designated site I between NADH and coenzyme Q, site II between cytochromes b and c, and site III between cytochrome a and free oxygen. Site III occurs within complex IV, the final complex which may also be referred to as cytochrome oxidase. Its overall function is to transfer elec-trons from cytochrome c to cytochrome a, then to a3 and finally to molecular oxygen. It is this final stage which is blocked by the action of cyanide and by carbon monoxide. Associated with the electron flow, is the ejection of hydrogen ions from inside the mitochondrion, across the membrane, and in complex IV, the reduction of the oxygen molecule with two hydrogen ions originating from inside the mitochondrion. If all three sites were involved, the amount of energy released is sufficient to drive the synthesis of two and a half molecules of ATP for each pair of electrons transported. If the first site was omitted, the number falls to one and a half. In neither case is it a complete integer because there is not a direct mole for mole relationship between electron transport and ATP synthesis but as described earlier, it is part of a much more complicated process described above as the chemiosmotic theory.
Bacterial electron transport systems and oxidative phosphorylation
Bacterial electron transport chains have fundamentally the same function as that described for mitochondrial electron transport chains but with several notable differences in their structure. For example, the cytochrome oxidase which is the final complex nearest the oxygen in mitochondria, is not present in all bacteria. The presence or absence of this complex is the basis of the ‘oxidase’ test for the identification of bacteria. In these organisms, cytochrome oxidase is replaced by a different set of cytochromes. An interesting example is Escherichia coli, an enteric bacterium and coliform, which is commonly found in sewage. It has replaced the electron carriers of cytochrome oxidase with a different set including cytochromes b558 , b595 , b562 , d and o, which are organised in response to the level of oxygen in the local environment. Unlike the mitochondrial chain, the bacterial systems may be highly branched and may have many more points for the entry of electrons into the chain and exit of electrons to the final electron acceptor.
Bacterial electron transport systems, denitrification and methanogenesis
As previously mentioned, the term respiration is applied to many processes. With-out further specification it is usually used to mean the consumption of molecular oxygen, by reduction to water in the case of the electron transport discussed above, or by oxidation of an organic molecule to produce carbon dioxide and ser-ine in the case of photorespiration, discussed later. Thus the term anaerobic respiration seems a contradiction. It does, however, describe funda-mentally the same process of electron transfer to a final acceptor which although inorganic, in this case is not oxygen. An example of such an electron acceptor is nitrate which is converted to nitrite. This is a toxic substance, and so many bacteria have the facility to convert nitrite to nitrogen gas. This overall series of reactions is described as denitrification and is the basis of the process by which denitrifying bacteria such as members of the Pseudomonas and Bacil-lus genera are able to reduce nitrate and nitrite levels down to consent valuesduring sewage treatment. Such bacteria have different components in their elec-tron transport chain in comparison with mitochondria, which have the necessary enzymatic activities to carry out these processes. Like mitochondrial electron transport, denitrification can be associated with synthesis of ATP although with much reduced efficiency.
Other examples of terminal electron acceptors are firstly sulphate, in which case one of the final products is elemental sulphur. This process is carried out by the obligate anaerobe, Desulfovibrio and members of the archaean genus Archaeglobus. Another anaerobe, Alkaliphilus transvaalensis, an extreme alka-liphile, growing at a pH of 8.5 to 12.5, isolated from an ultra-deep gold mine in South Africa, can use elemental sulphur, thiosulphate or fumarate as an additional electron acceptor (Takai et al. 2001). Secondly, carbon dioxide may be the final electron acceptor in which case one of the final products is methane. This pro-cess is also carried out by obligate anaerobes, in this case, the methanogens, all of which are archaeans and are responsible for methane production in anaerobic digesters and landfill sites. Again, it functions on much the same principles as the other chains mentioned above but has a different set of cofactors which are most unusual. For both of the above obligate anaerobes, anaerobic respiration is an important mechanism of ATP synthesis. It is less efficient than aerobic respiration due to the smaller drop in electropotential between sulphate or carbon dioxide and NADH compared with the difference between NADH and oxygen, and so less energy is available to be released during electron transport and consequently less ATP is synthesised per mole of NADH entering the pathway. Anaerobic respiration is, however, more efficient than fermentation and so is the route of choice for ATP synthesis for an anaerobe.
The energy balance sheet between substrate level and electron transport linked ATP synthesis
An approximate comparison may be made between the efficiency with respect to energy production, of ATP synthesis by substrate-level phosphorylation and by association with electron transport. For one mole of glucose passing through glycolysis by the Embden – Meyerhof pathway to produce two moles of pyruvate, there is net production of two moles of ATP. For most fermentation pathways, no further ATP is synthesised. There are exceptions, of course, such as the con-version of an acyl CoA derivative such as acetyl CoA or butyryl CoA to the free acid which in these cases are acetate and butyrate respectively. Each of these reactions releases sufficient energy to drive the phosphorylation of one mole of ADP. Conversely, if the electron transport chain is functioning, NADH may be oxidised by relinquishing electrons to the cytochromes in the chain thus regener-ating the oxidised cofactor. In this scenario, pyruvate may enter the TCA cycle rather than a fermentation route, thus a further mole of ATP is produced at sub-strate level during conversion of succinyl CoA to succinate via GTP, which then transfers the terminal phosphate to ATP. In addition, NADH and FADH2 are pro-duced during the TCA cycle thus generating up to 15 moles of ATP per mole of pyruvate. An overall comparison may be made between glycolysis followed by reoxidation of NADH by fermentation or, alternatively, glycolysis followed by entry into the TCA cycle and reoxidation of cofactors via the electron transport chain. Remembering that one mole of glucose generates two moles of pyruvate during glycolysis, and that the two moles of NADH produced during glycoly-sis may also be reoxidised by transfer to the electron transport chain and not through fermentation, the net result is that glucose catabolised by the glycoly-sis – fermentation route results in the production of two moles of ATP whereas catabolism by the glycolysis – TCA cycle – electron transport/oxidative phospho-rylation route produces up to 32 moles of ATP. The figure of 36 was deduced by Lehninger (1975) but has been revised more recently to reflect the tenets of the chemiosmotic theory described earlier.
Anaerobic respiration is less efficient than aerobic respiration. Oxidation of the same amount of cofactor by methanogenesis rather than oxidative phospho-rylation would produce fewer moles of ATP. Consequently, for a given amount of ATP production, the flux of glucose through glycolysis followed by fermen-tation would have to be approximately 16 times greater than through glycolysis followed by oxidative phosphorylation, and the flux through methanogenesis is somewhat intermediate. It is the metabolic capability of the organism and the presence or absence of the appropriate inorganic electron acceptor which deter-mines the fate of pyruvate on the grounds of energy considerations. On a practical basis this may explain why anaerobic processes, such as the anaerobic digestion of sewage sludge and municipal solid waste, are considerably less exothermic than their aerobic counterparts. For a given quantity of carbon source, an aerobic process will be able to extract in the order of 10 times the amount of energy than that generated by an anaerobic process.
Regeneration of NAD+ in plants
In addition to the processes discussed above for the production of NADH, plant mitochondria operate an additional system whereby the required protons are derived from two molecules of the amino acid glycine. During this mitochon-drial process, one molecule of molecular oxygen is consumed in the production of carbon dioxide and the amino acid, serine. The superfluous amino group from the second glycine molecule is released as ammonia. The glycine molecules were derived from phosphoglycolate, the metabolically useless product of pho-torespiration. This subject is very important with regard to plant breeding and development and so is discussed in some detail alongside the related subject of photosynthesis.
Related Topics