Chapter: Basic Radiology : Radiology of the Urinary Tract
Exercise: Renal Mass
EXERCISE 9-2.
RENAL MASS
9-4. Based on the MR
images for Case 9-4 (Figure 9-21), what is the most likely diagnosis?
A.
A dromedary hump
B.
A malignant primary renal neoplasm
C.
A simple renal cyst
D.
A metastatic lesion from a distant primary malig-nancy.
9-5. Which is not true of the lesion shown in Case 9-5
(Figure 9-22)?
A.
This lesion contains fat.
B.
CT is the key to definitive diagnosis.
C.
The ultrasound finding is nonspecific.
D.
The lesion shown is the most common malignant renal neoplasm.
9-6. Which of the
following is not true of the lesion
seen in Case 9-6 (Figure 9-23)?
A.
This is the most common primary renal malig-nancy.
B.
This lesion is classically associated with the clini-cal triad
of flank pain, hematuria, and a palpable mass.
C.
This type of lesion often contains fat.
D.
This lesion does not enhance with IV contrast.
9-7. How can one
differentiate the lesion in Case 9-7 (Figure 9-24) from that seen in Case 9-6?
A.
By CT densitometry
B.
By ultrasonographic characteristics
C.
These lesions cannot be distinguished by imaging.
D.
By MR signal characteristics
Radiologic Findings
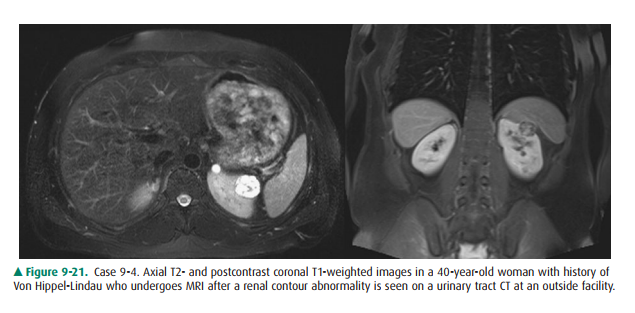
Regarding Case 9-4 (Figure 9-21),
the MRI images show a rounded, exophytic T2-intense lesion with heterogeneous
in-ternal signal on T1 sequences. This appearance is highly sus-picious for
renal cell carcinoma (B is the correct answer to Question 9-4). Patients with
VHL (Von Hippel-Lindau) have a 70% risk of developing renal cell carcinoma by
60 years of age. This lesion clearly differs in signal characteristics
fromnormal renal parenchyma (A is incorrect). Renal cysts would be homogenously
T2 intense, with only thin septation at present, and would not demonstrate
diffusely heterogeneous T1 signal (C is incorrect). A metastasis could imitate
renal cell carcinoma (RCC) in signal, but would be less likely (D is
incorrect).
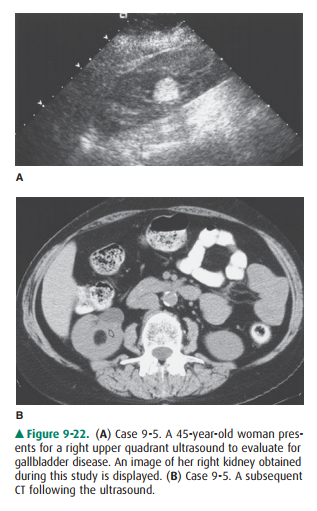
In Case 9-5, the ultrasound image
(Figure 9-22 A) reveals a hyperechoic lesion with echogenicity similar to that
of adja-cent perirenal fat. However, this appearance on ultrasound is
nonspecific and requires further evaluation, and a CT scan should generally be
obtained. The CT results (Figure 9-22 B) show that this lesion (arrow) does
indeed contain fat. The presence of definitive fat within a renal mass is
virtually pathognomonic for the diagnosis of angiomyolipoma (AML), which is a
benign lesion containing fat, blood vessels, and smooth muscle (D is the
correct answer to Question 9-5).
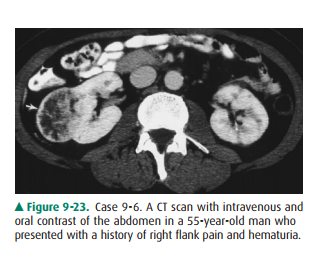
For Case 9-6, the lesion seen
(Figure 9-23) is an inho-mogenous soft-tissue mass (arrow) arising from the
right kidney, which proved to be a renal cell carcinoma. It displays many of
the common CT characteristics of RCC, including a somewhat rounded shape with
irregular margins, enhance-ment with IV contrast material, and inhomogeneity
(which can be due to hemorrhage, proteinaceous debris, and even
calcifications). Renal cell carcinomas almost never contain fat (C is the
correct answer to Question 9-6).
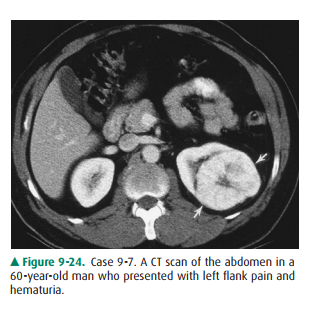
Regarding Case 9-7, the lesion
has imaging and clinical characteristics indistinguishable from those of RCC.
How-ever, the lesion (arrows) (Figure 9-24) is an oncocytoma, a benign tumor
arising from the distal tubule or collecting ducts. Classically, oncocytoma is
often associated with a characteristic central stellate scar. However, scarring
can be seen in a RCC as well, and these lesions cannot be reliablydistinguished
by imaging alone (C is the correct answer to Question 9-7).
Discussion
These cases demonstrate examples
of the most common renal masses, both benign and malignant. In general, all of
these renal masses expand and displace normal renal parenchyma and nor-mal
collecting-system structures. They are distinguished from infiltrating
processes (such as infiltrating neoplasms, infections, and infarctions), which
tend to preserve normal renal mor-phology. Expansile or exophytic renal masses
may be seen by cross-sectional imaging and occasionally by conventional
radi-ography if the mass is large. Imaging is used primarily to differ-entiate
between those lesions that are clearly benign, those that are probably benign
but require surveillance, and those that may be malignant and require tissue
diagnosis.
The simple cyst is the most
common renal mass, present in up to 50% of the population over the age of 50.
They are almost always asymptomatic and discovered incidentally. Although they
occasionally may become infected, hemor-rhage, or cause pain, their main
importance lies in differenti-ating the lesions from renal tumors. Cysts can be
single or multiple, unilateral or bilateral, and vary greatly in size.
Pathologically they are thought to be acquired lesions arising from blocked
collecting ducts or tubules. They have thin fi-brous capsules lined with epithelial
cells and contain clear serous fluid. Only the largest of renal cysts may be
evident on plain x-rays. On cross-sectional imaging modalities, cysts are
sharply demarcated from adjacent parenchyma, homoge-nous in appearance, rounded
with imperceptible walls, and do not enhance with the administration of
contrast material. By ultrasound, a clearly anechoic lesion demonstrating
en-hanced through-transmission of sound as well as the forego-ing criteria can
be diagnosed as a simple cyst. On unenhanced CT, cysts measure near water
density ( 10 HU). No mural nodularity or thick septation should be seen by CT.
Internal hemorrhage often makes cysts appear complex, and in these cases,
contrast must be administered to evaluate for enhancement that would indicate a
mass. An exception is in homogenous lesions measuring greater than 70 HU in
attenu-ation. Recent studies have indicated that these hyperdense le-sions
almost always represent a hyperdense cyst. Lesions not fulfilling these
criteria for cyst, such as those with thick enhanc-ing walls, those containing
internal debris, or those with calcifi-cations, may represent cystic neoplasms
and must be evaluated further by serial imaging and/or histological diagnosis.
Solid renal masses are of even
greater concern. One such le-sion, the angiomyolipoma, is most easily
distinguished from other renal masses by the presence of internal fat. These
lesions are hamartomatous tumors of mesenchymal origin that are usually well
differentiated and benign. In addition to fat, they contain sheets of smooth
muscle and thick-walled blood ves-sels. Incidence is highest in middle-aged
females. Although these are usually asymptomatic, they are predisposed to
spon-taneous hemorrhage, especially when large. They can occur as sporadic solitary
lesions or in association with tuberous sclero-sis, in which case multiple AMLs
are often present. Twenty per-cent of patients with an AML will have tuberous
sclerosis, and up to 80% of patients with tuberous sclerosis will have an AML.
Macroscopic fat in a renal mass by CT is essentially diag-nostic of AML.
Although these lesions are benign, they are often removed when greater than 4
to 5 cm because of the in-creased risk of hemorrhage, and for this reason
smaller AMLs require follow-up to monitor the lesion for growth.
Another benign renal neoplasm
that deserves comment is the oncocytoma, which originates from the epithelium
of the distal tubules or collecting ducts. A central stellate scar is a
characteristic, although nonspecific, pathologic feature of these lesions. They
are typically asymptomatic and discov-ered incidentally, though they
occasionally may be associated with a flank mass, pain, or hematuria. On
cross-sectional im-aging studies, oncocytomas appear as a well-defined renal
mass. The diagnosis of oncocytoma may be suggested by a central stellate scar.
However, even when classic, the imaging characteristics described earlier
cannot be used to reliably differentiate them from malignant renal cell
carcinoma, and excision is generally indicated. Note that biopsy is usually not
recommended because the cytologic appearance of oncocy-toma and renal cell
carcinoma may be indistinguishable.
Renal cell carcinoma (RCC) is the
most common primary renal malignancy, originating from the epithelium of the
proximal tubule, having a male predominance and a peak in-cidence in adults in
their 50s. Any renal mass lesion that can-not be definitively identified as one
of the benign entities mentioned earlier must be assumed to be RCC until proven
otherwise, most often by tissue diagnosis. Classically, RCC is associated with
the clinical triad mentioned earlier of flank pain, a flank mass, and
hematuria, although all three are pres-ent in less than 10% of cases. More
commonly, these lesions are being discovered incidentally before symptoms have
de-veloped. They may demonstrate calcifications in up to 30% of cases. On
ultrasound, a nonspecific renal mass is seen. RCC may be hyperechoic and mimic
AML or have central necrosis mimicking the central scar of oncocytomas. By CT,
they tend to be rounded soft-tissue masses, enhancing after the administration
of IV contrast. When small, they are often homogeneous, though when larger they
are more heteroge-neous, frequently with necrosis and often with
calcifications. One important role for imaging beyond detecting renal cell
carcinoma is evaluating the extent of tumor spread. RCC can extend locally and
invade adjacent soft tissues, especially when large and extensive. In addition,
renal cell carcinoma has a propensity to spread into the renal veins and
beyond, and the extent of this must be delineated prior to surgery. En-larged
lymph nodes and spread to liver, lung, bones, and other areas, suggesting
metastatic disease, should be sought. Surgi-cal excision is the treatment of
choice for resectable lesions, making accurate staging to determine surgical
candidacy all the more important.
Related Topics