Chapter: Basic Radiology : Radiology of the Urinary Tract
Computed Tomography - Radiology of the Urinary Tract: Techniques and Normal Anatomy
Computed Tomography
Multidetector (spiral) CT is now
the dominant radiologic imaging modality for evaluation of the urinary tract
and ad-renal glands. Several factors make CT quite effective. The high contrast
and spatial resolution afforded by CT allow de-tection and evaluation of subtle
differences in very small structures. Mathematical calculations of the
attenuation of the CT x-ray beam allow quantitative evaluation of the rela-tive
density of structures (ie, their Hounsfield units) and by using these “CT
numbers,” much unique diagnostic informa-tion on the urinary tract is gained.
Examinations can be per-formed quickly and reproducibly with thin CT slices of
the entire urinary tract now obtainable in just a few seconds. With these
advances, CT can now be used to evaluate much of the urinary tract, including
vascular, parenchymal, and urothelial components as well as adjacent structures
including the adrenal glands.
Careful techniques and protocols
are critical to CT accu-racy. CT scans of the urinary tract may be performed
with and/or without intravenous iodinated contrast material de-pending on the
indications. CT performed without contrast is typically used for the detection
of renal or ureteral calculi, for which it is exquisitely sensitive.
Additionally, noncontrast views of the kidneys serve as a baseline to evaluate
for lesion enhancement after contrast administration, a critical factor in mass
evaluation. Intravenously administered iodinated contrast is excreted by the
kidney primarily by glomerular fil-tration, opacifying the urinary tract
progressively from thekidney through the ureter and to the bladder. Contrast
“opacification” during CT is most accurate, exquisitely demon-strating and
evaluating the urinary tract. One of the major advances in the past 5 to 10
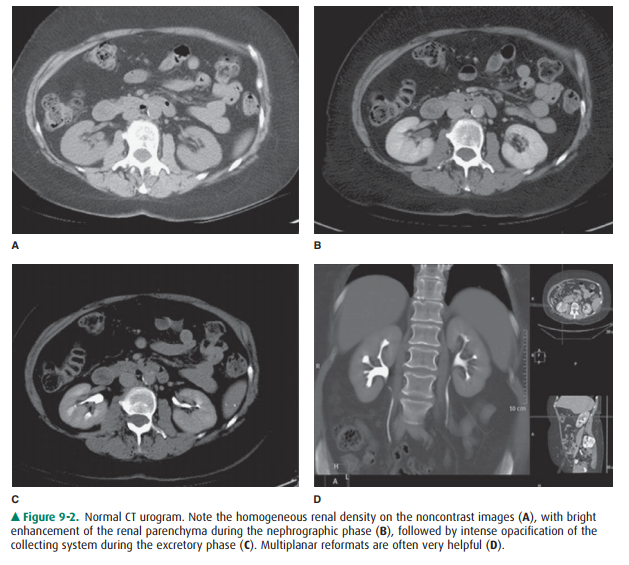
years has been advent of CT urography (CTU), a superior replacement of the IVP.
CTU is most often indicated for evaluation of hematuria and typi-cally consists
of three scanning phases—noncontrast, nephrographic (90 seconds), and delayed
(8 to 10 minutes) excretory phase. The noncontrast phase allows for stone
de-tection and serves as a baseline to assess possible mass en-hancement. The
nephrographic phase is predominately used to evaluate the kidneys for mass
lesions. Finally, the excretoryphase allows assessment of the collecting
system, particularly for the detection of urothelial carcinoma (Figure 9-2).
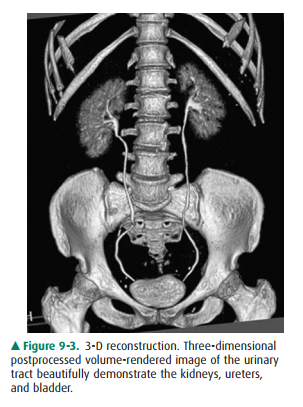
Fre-quently, the axial CT images are augmented with multiplanar and
three-dimensional reconstructions (Figure 9-3).
The kidneys, which enclose the
renal sinus and are sur-rounded by retroperitoneal fat, are well delineated on
CT. The renal parenchyma is composed of outer cortex, contain-ing much of the
nephron, as well as the pyramid-shaped col-lecting duct containing inner
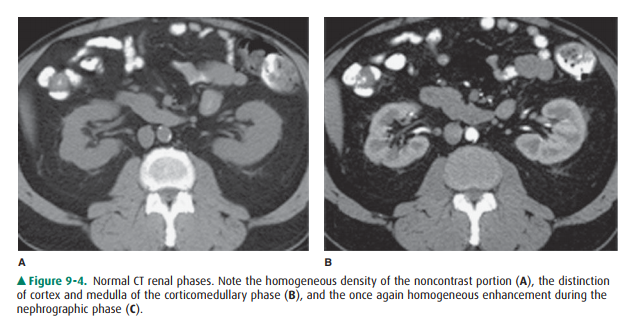
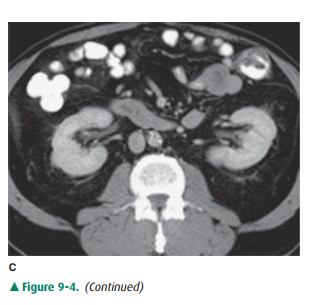
medulla. On noncontrast examinations, the kidneys are homogeneous and have a
den-sity similar to most soft tissue (Figure 9-4 A). With rapid scanning after
contrast administration, several sequentialphases of opacification within the
kidney can be delineated by CT including the corticomedullary, nephrographic,
and excretory phases. The corticomedullary phase can be seen if scanning is
performed during the first 20 to 70 seconds after contrast administration and
represents the early preferential blood flow to the renal cortex (Figure 9-4
B). Subsequently, contrast begins to pass into the distal collecting tubules
within the renal medulla, resulting in a more homogeneous opacification of the
renal parenchyma termed the CT nephrographic phase (Figure 9-4 C). This
generally occurs around 90 to 120 seconds after contrast medium injection.
Finally, the excretory phase is seen when contrast opacifies the collecting
system. Each different phase of opacification may better demonstrate different
disease processes, and thus various scanning protocols are used to evaluate the
kidneys depending on the clinical indication.
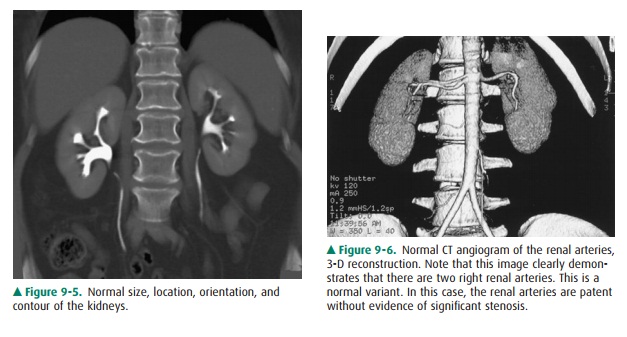
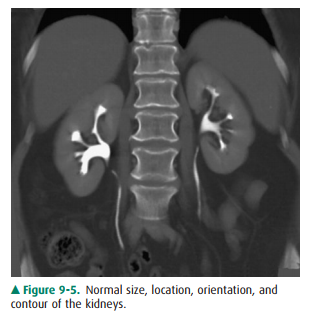
On CT, the kidneys should be
evaluated for size, location, orientation, and contour (Figure 9-5). The
kidneys are typi-cally located at the level of the upper lumbar spine with the
right kidney slightly lower than the left. They generally lie with their axes
along the psoas muscles with the upper pole slightly more medial than the lower
pole. Alterations in position and orientation of the kidneys may be related to
congenital anomalies such as pelvic kidneys or may be sec-ondary to mass effect
from an adjacent lesion. The size of the kidneys is somewhat variable depending
on the age, sex, and size of the patient, but generally range from 11 to 14 cm.
Al-though the right is often slightly smaller than the left, the kid-neys
should be relatively symmetric in size, with a discrepancygreater than 2 cm
suggesting pathology. There are a number of causes of abnormal renal size,
ranging from incidental anomalies such as congenital renal hypoplasia to
clinically significant conditions such as renal artery stenosis (small kid-ney)
or infiltrating renal neoplasm (large kidney). The kid-neys should have a
reniform shape and a smooth contour. Clefts suggest scarring, which most often
results from chronic vesicoureteral reflux/chronic bacterial pyelonephritis or
fromrenal infarcts. Additionally, the kidneys should be evaluated for
calcifications, hydronephrosis, and inflammation. A criti-cal role for CT is
mass/cyst detection and characterization. CT is very specific in identifying a
lesion as a simple cyst when the lesion is homogenous and of water density,
typi-cally 10 HU. Lesions of higher density may represent hy-perdense (complex)
cysts or solid masses, and further evaluation with contrast CT to detect
enhancement may be needed to differentiate these causes. Fat within a solid
mass generally allows a confident diagnosis of angiomyolipoma. A solid,
non-fat-containing mass in the adult should be consid-ered a renal cell
carcinoma until proven otherwise. CT is also useful in staging renal neoplasms.
Non-neoplastic renal dis-ease, such as trauma and complicated infections, is
accurately demonstrated on CT, providing specific information regard-ing the
extent and severity of the process. The remainder of the retroperitoneum,
containing fat, the normal occupants of the retroperitoneum (kidneys, adrenal
glands, pancreas, duo-denum, and parts of the colon), and vascular structures,
is well seen by CT, and diseases such as inflammation, infection, and neoplasms
are readily demonstrated. Additionally, the thin section and rapid imaging of
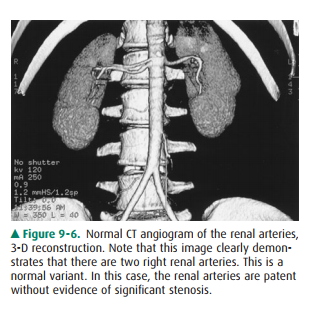
modern CT also allows noninvasive evaluation of the vascular system, including
the major renal arterial and venous structures (Figure 9-6).
As previously mentioned, the
unenhanced CT of the uri-nary system, commonly referred to as the urinary tract
CT or “stone study,” is the procedure of choice for the detection of urolithiasis
and associated obstruction, with unmatched specificity and sensitivity. There
are many other advantagesto using CT to evaluate suspected ureteral stones,
including speed of the examination, identification of alternative expla-nations
for the pain (appendicitis, diverticulitis, aneurysm, etc), and elimination of
intravenous contrast complications. Scans no thicker than 5 mm are performed
from the top of the kidneys to the symphysis pubis. The ureter can be
visual-ized and followed from the renal pelvis to the bladder in most cases and
appears as a tubular 2- to 3-mm fluid structure sur-rounded by retroperitoneal
fat. Stones can be diagnosed by their high density and location within the
ureter. Secondary signs of obstruction have been described, including dilation
of the proximal ureter. As on the KUB, phleboliths can prove troubling because
of their frequent close approximation with the distal ureter; however, their
central lucency, lack of sur-rounding inflamed ureteral wall, and lack of
secondary signs of obstruction usually allow their distinction.
Although visible without contrast
material, the intrarenal collecting system, ureters, and bladder are
conspicuous and nicely demonstrated when contrast material has been
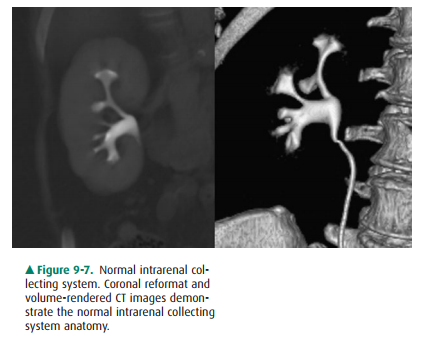
admin-istered and delayed images obtained. Collecting system anatomy is now
visible in fine detail with the use of modern high-resolution ( 1 mm) spiral CT
and multiplanar (coro-nal, sagittal) and three-dimensional reformat methods.
The intrarenal collecting system consists of calyces, infundibula, and the
renal pelvis. Normally, each kidney consists of 7 to 14 evenly distributed
calyces (Figure 9-7). The individual renal calyx, from the Latin for “chalice,”
is a delicate-appearing cup-shaped structure. Not uncommonly, partial fusion of
the calyces occurs, especially in the renal poles, creating the com-pound
calyx. Other calyceal variants occur, including vari-ants of number
(polycalycosis, unicalyx kidney) and size (megacalycosis, microcalyx) and must
be differentiated from true pathology. The normal delicate, cuplike appearance
can be distorted or irregular in conditions such as papillary necrosis,
tuberculosis, or urothelial (transitional cell) carci-noma. Diverticula may
arise from the calyces creating a haven for stone formation, recurrent
infection, or even transitional-cell malignancy. The renal pelvis is also quite
variable in ap-pearance. A common variation is the so-called extrarenal pelvis,
where the pelvis lies outside the renal sinus. In this set-ting, the pelvis
tends to be more prominent and rounded, mimicking hydronephrosis. This can be
differentiated from true obstruction by normal-appearing calyces. The ureters
appear as contrast-containing, rounded structures in the retroperitoneum
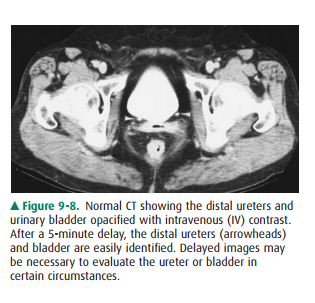
(Figure 9-8). Because of peristalsis, portions of the ureters may be collapsed.
On CT, the bladder appears as a rounded water or contrast density structure in
the pelvis. Detection of malignancy arising from the urinary tract epithe-lium
is a major concern, particularly with the advent of CT urography. Urothelial
neoplasia generally appears as papillary solid lesions arising from the
urothelial mucosa. Occasionally, urothelial neoplasm may appear as a more flat
area of mucosal thickening. The urethra is not normally seen on CT.
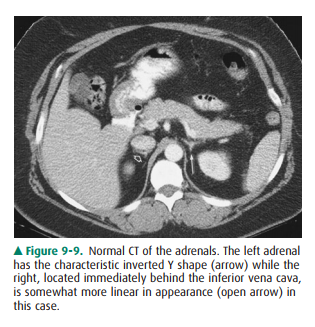
The adrenal glands are well seen
on CT, appearing as linear, arrowhead, or inverted Y-shaped structures usually
consisting of body and medial and lateral limbs (Figure 9-9). The adrenal
glands are normally less than 1 cm in thickness and 3 to 4 cmin length. They
lie cephalad to the kidneys, with the right just posterior to the inferior vena
cava (IVC) and the left antero-medial to the upper pole of the left kidney.
Note that the em-bryological and functionally distinct outer cortex and inner
medulla are not visibly separable. Although occasionally af-fected by trauma or
infection, the adrenal mass evokes the most attention, and for this CT
evaluation is well suited.
Related Topics