Chapter: Biology of Disease: Disorders of Water, Electrolytes and Urate Balances
Disorders of Ca2+ Homeostasis
DISORDERS OF Ca2+ HOMEOSTASIS
Calcium is required for bone and teeth structure, the release of
neuro-transmitters and initiation of muscle contraction, as a cofactor for
coagulation factors , some enzyme activities and it also acts as an
intracellular second messenger for a number of hormones .
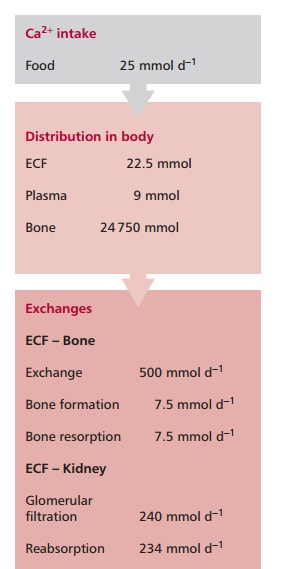
The normal dietary intake of Ca2+of about 25 mmol day–1
is supplemented by the reabsorption of Ca2+ from gastrointestinal
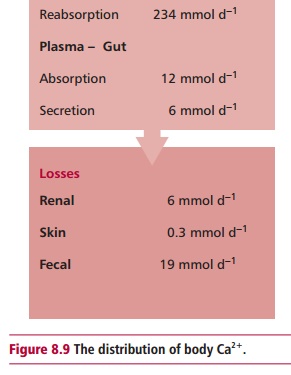
secretions. Approximately 19 mmol of Ca2+ is lost in the feces
daily. The kidneys normally filter about 240 mmol of Ca2+ daily but,
as most of this is reabsorbed by the tubules, normal renal loss of Ca2+
is only about 6 mmol per day (Figure 8.9).
Calcium is the most abundant mineral in the body and the average adult contains
approximately 1 kg or 25 000 mmol of Ca2+. Approximately 99% of Ca2+
is present in the bone. About 500 mmol of Ca2+ is exchanged daily
between bone and the ECF. The ECF contains about 22.5 mmol of Ca2+,
of which 9.0 mmol is present in the plasma. Approximately 47% of Ca2+
in plasma occurs as free ionized Ca2+, 46% is protein bound and 7%
is complexed with citrate or phosphate. Only free Ca2+ is
physiologically active and its plasma concentration is controlled by
homeostatic mechanisms involving the hormones parathyroid hormone (PTH),
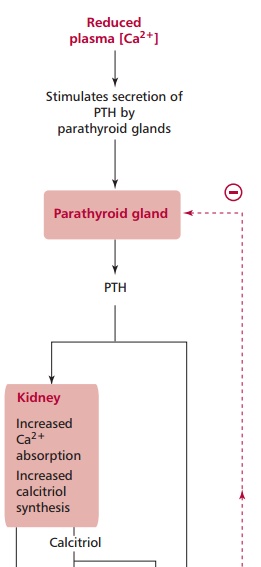
calcitriol and calcitonin (Figure 8.10).
Parathyroid hormone is secreted by the parathyroid glands in response to a fall
in the concentration of plasma ionized Ca2+ and vice versa. It stimulates the release of Ca2+ from bone,
a process called bone resorption,
and a decreased reabsorption of HCO3– by the kidneys that
produces an acidosis, which helps to increase plasma ionized Ca2+
and stimulates the synthesis of calcitriol from cholecalciferol in the liver.
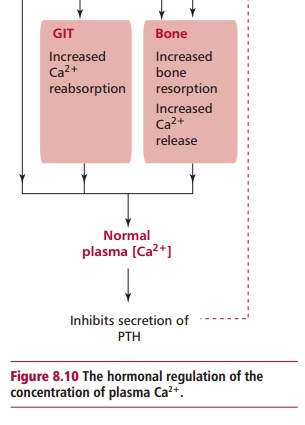
This hormone is also formed in the skin by the action of ultraviolet
light on 7-dehydrocholesterol. Calcitriol increases Ca2+ and Pi
absorption from the GIT and increases bone resorption. The physiological
function of calcitonin remains unclear but it is known to reduce the
concentration of Ca2+ in plasma by inhibiting both bone resorption
and the renal reabsorption of Ca2+.
The serum reference range for total Ca2+ is 2.20–2.60
mmol dm–3 and for free 1.20–1.37 mmol dm–3. Values above
and below these are called hypercalcemia and hypocalcemia respectively.
The renal damage associated with hypercalcemia is its most
serious consequence. Hypercalcemia may suppress neuromuscular excitability
causing constipation and abdominal pain and affect the CNS, resulting in
depression, nausea and anorexia. The nausea may cause vomiting and therefore
dehydration. Calcium can stimulate gastrin and therefore gastric acid secretion
and so hypercalcemia may be associated with peptic ulcers . Hypercalcemia may
cause arrhythmias and in severe cases may result in cardiac arrest . The
commonest causes of hypercalcemia are malignant disease or primary
hyperparathyroidism. Less common causes include thyrotoxicosis, vitamin D
intoxication, thiazide diuretics and familial hypocalciuric hypercalcemia. Rare
causes are tuberculosis, sarcoidosis, acromegaly, milk-alkali syndrome and
idiopathic hypercalcemia of infancy.
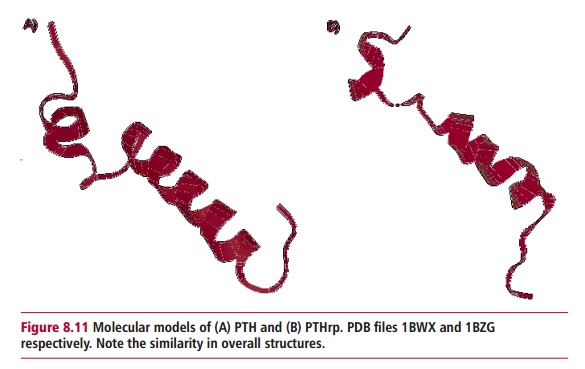
Cancerous tumors of the lungs stimulate an increase in plasma Ca2+
by producing a PTH related protein (PTHrp) that resembles the structure of PTH
(Figure 8.11). Cytokines and prostaglandins
released by tumors that have metastasized to the bones, may lead to increased
resorption of Ca2.
Primary hyperparathyroidism occurs most commonly due to a parathyroid adenoma,
which is a benign tumor, and only rarely due to a parathyroid carcinoma. It
affects both men and women at any age but is most common in postmenopausal
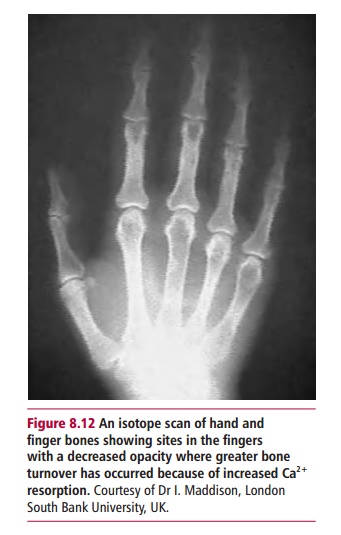
women. In primary hyperparathyroidism, there is excessive PTH secretion that
causes hypercalcemia and sometimes hypophosphatemia, which increases bone
turnover particularly of the metaphyses (Figure
8.12). Thyroid hormones have no direct effect on Ca2+
homeostasis but can cause increased bone turnover by increasing osteoclastic
activity and giving rise to mild hypercalcemia during thyrotoxicosis. An
excessive iatrogenic or accidental ingestion of vitamin D or thiazide diuretics
that interfere with renal Ca2+ loss can also cause hypercalcemia.
Familial hypocalciuric hypercalcemia is a recently recognized autosomal dominant condition that develops from childhood. It is characterized by chronic hypercalcemia but is usually
asymptomatic, with normal levels of PTH and no parathyroid adenoma. The
mechanism underlying this condition is unknown. Both sarcoidosis and
tuberculosis are granulomatous diseases. In these conditions, hypercalcemia
occurs as there is increased production of calcitriol by macrophages in the
granulomas. Hypercalcemia is occasionally seen in acromegaly, probably due to
stimulation of calcitriol production by excess growth hormone. Hypercalcemia may
occur in people who ingest large amounts of milk together with alkali antacids,
such as HCO3–, to relieve symptoms of peptic ulceration.
An alkalosis occurs that is believed to reduce renal Ca2+ excretion
although the precise mechanism is still unclear. This milk-alkali syndrome is
very rare as antacid treatment of peptic ulcers has been replaced by drugs that
inhibit gastric acid secretion. The condition idiopathic hypercalcemia of
infancy is associated with hypercalcemia because of an increased sensitivity to
vitamin D in bone and the GIT but the precise mechanism underlying this
hypercalcemia is unknown.
Patients who present with hypercalcemia are investigated for
malignancy or primary hyperparathyroidism as this accounts for up to 90% of
cases. If both malignancy and primary hyperparathyroidism are excluded, other
causes must be considered and investigated. A number
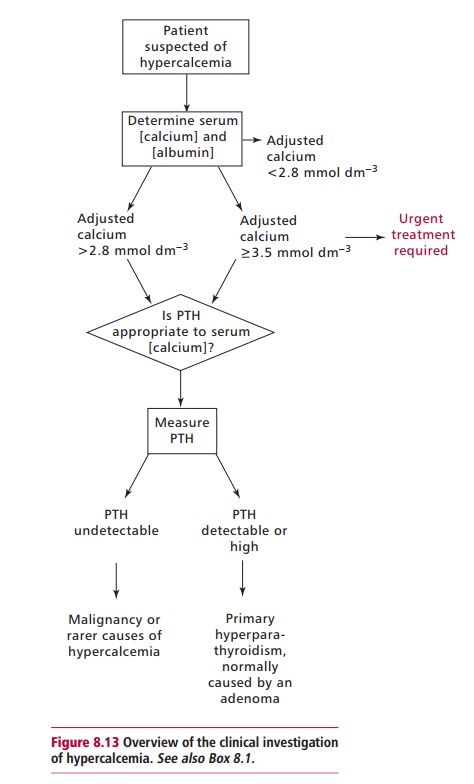
of approaches are taken to managing hypercalcemia. The
underlying cause should be treated wherever possible. Intravenous saline may be
administered in dehydrated patients to restore the glomerular filtration rate
and enhance Ca2+ loss and hydration. Drugs, such as frusemide,
inhibit renal reabsorption of Ca2+ and promote its excretion while
bisphosphonates lower Ca2+ levels by inhibiting bone resorption. In
very severe cases, dialysis or emergency parathyroidectomy may be necessary. In
some cases, an artefactual hypocalcemia may be reported when blood samples are
erroneously collected into tubes containing ethylene diaminetetraacetic acid
(EDTA). This anticoagulant is a chelator of Ca2+ and its use will
lead to low values for Ca2+ concentrations.
The clinical effects of hypocalcemia include behavioral
disturbances, paresthesiae, tetany, convulsions and cataracts. Its major causes
are renal failure, Mg2+ and vitamin D deficiencies,
hypoparathyroidism and pseudohypoparathyroidism. Chronic renal failure may
decrease the reabsorption of Ca2+ by decreasing the synthesis of
calcitriol leading to hypocalcemia. This may lead to bone disease because the
increased output of PTH arising from the hypocalcemia can increase osteoclast
activity. Magnesium ions are required for PTH secretion and its action and a
deficiency produces hypocalcemia. A deficiency in vitamin D may arise from a poor
diet, malabsorption or inadequate exposure to sunlight leading to an inadequate
absorption of Ca2+ from food. Hypoparathyroidism, or a reduced
activity of the parathyroid glands with decreased production of PTH, results in
hypocalcemia. The condition can be congenital, where there is an absence of the
parathyroid glands, or acquired hypoparathyroidism that may be idiopathic, or
caused by autoimmune conditions or surgery, for example thyroidectomy. In
pseudohypoparathyroidism, there is excessive PTH secretion because target
tissues fail to respond to the hormone, producing a persistent hypocalcemia. This
condition is more common in males than females and patients present with
skeletal abnormalities including short stature, mental retardation, cataracts
and testicular atrophy.
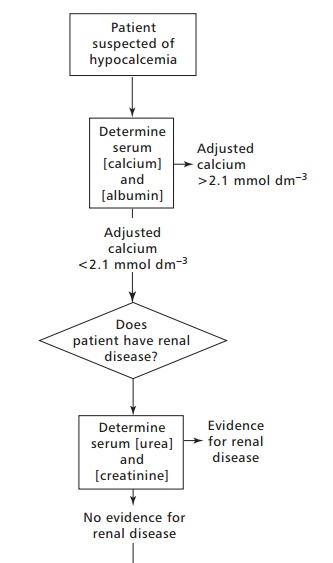
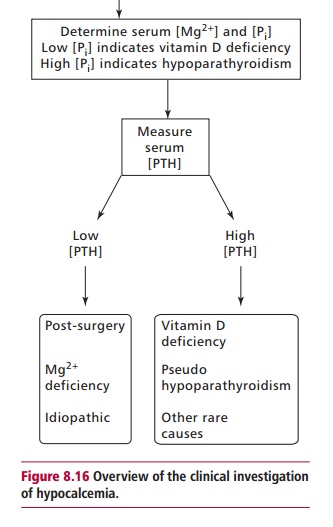
The investigation of hypocalcemia is outlined in Figure 8.16. The underlying cause of
hypocalcemia should be treated wherever possible. Magnesium supplements may be
prescribed in hypocalcemia due to Mg2+ deficiency, whereas
calcitriol and its precursors may be prescribed in vitamin D deficiency. Oral
Ca2+ supplements are prescribed in mild cases of hypocalcemia.
Related Topics