Chapter: Basic & Clinical Pharmacology : Development & Regulation of Drugs
Development & Regulation of Drugs
Development & Regulation of
Drugs
A
few useful drugs have been known since humans first began ingesting or injecting
substances and recording the results (see The History of Pharmacology), but the
majority of agents in current use have been developed during the last 100 years
using a variety of pharmacologic and toxicologic techniques. These new
chemicals and the efforts to market them have in turn led to a variety of
methods of legal regulation. The most common first steps in the development of
a new drug are the discovery or synthesis of a potential new drug com-pound or
the elucidation of a new drug target. When a new drug molecule is synthesized
or discovered, subsequent steps seek an understanding of the drug’s
interactions with its biologic targets. Repeated application of this approach
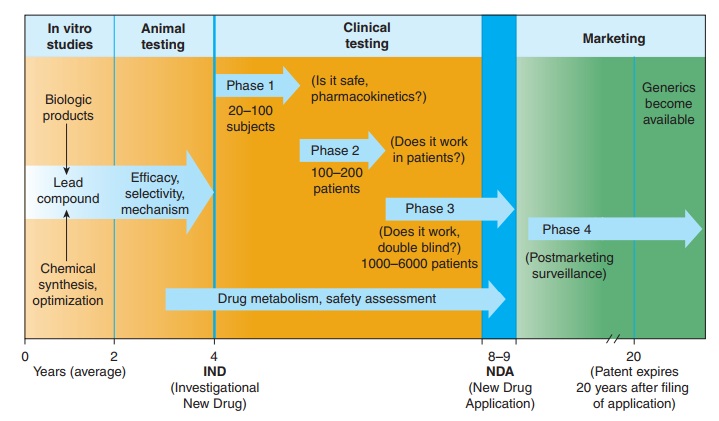
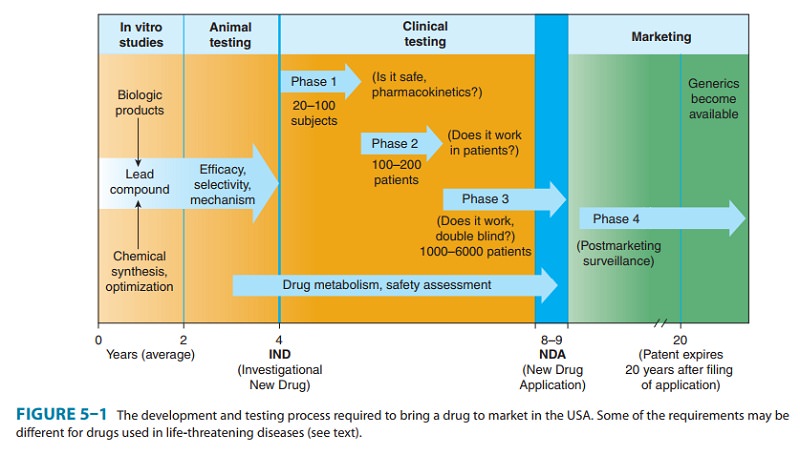
leads to compounds with increased efficacy, potency, and selectivity (Figure 5–1).
In the United States, the safety and efficacy of drugs must be defined before
marketing can be legally carried out. In addition to in vitro studies, relevant
biologic effects, drug metabolism, pharmacoki-netic profiles, and particularly
an assessment of the relative safety of the drug must be characterized in vivo
in animals before human drug trials can be started. With regulatory approval,
human test-ing may then go forward (usually in three phases) before the drug is
considered for approval for general use. A fourth phase of data gathering and
safety monitoring is becoming increasingly impor-tant and follows after
approval for marketing. Once approved, the great majority of drugs become
available for use by any appropri-ately licensed practitioner. Highly toxic
drugs that are nevertheless considered valuable in lethal diseases may be
approved for restricted use by practitioners who have undergone special
train-ing in their use and who maintain detailed records.
Related Topics