Chapter: Basic & Clinical Pharmacology : Development & Regulation of Drugs
Preclinical Safety & Toxicity Testing
PRECLINICAL SAFETY & TOXICITY
TESTING
All drugs are toxic in some individuals at
some dose. Seeking
to cor-rectly define the limiting toxicities of drugs and the therapeutic index
comparing benefits and risks of a new drug is an essential part of the new drug
development process. Most drug candidates fail to reach the market, but the art
of drug development consists of effective assessment and management of risk
versus benefit and not total risk avoidance.Candidate drugs that survive the
initial screening procedures must be carefully evaluated for potential risks
before and during clinical testing. Depending on the proposed use of the drug,
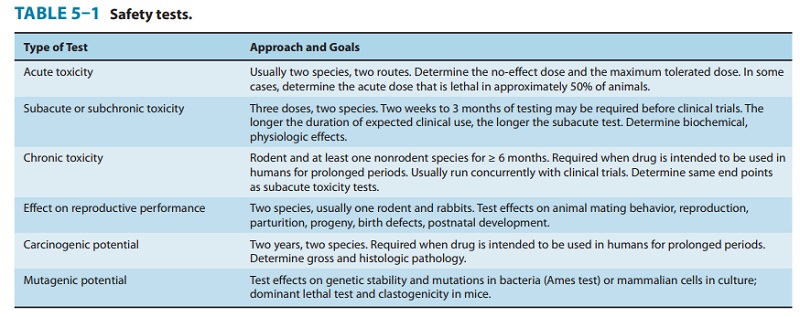
pre-clinical toxicity testing includes most or all of the procedures shown in
Table 5–1. Although no chemical can be certified as completely “safe” (free of
risk), the objective is to estimate the risk associated with exposure to the
drug candidate and to consider this in the context of therapeutic needs and likely
duration of drug use.The goals of preclinical toxicity studies include
identifying potential human toxicities, designing tests to further define the
toxic mechanisms, and predicting the most relevant toxicities to be monitored
in clinical trials. In addition to the studies shown in Table 5–1, several
quantitative estimates are desirable. These include the no-effect dose—the maximum dose at which a speci-fied toxic effect
is not seen; the minimum lethal dose—the
small-est dose that is observed to kill any experimental animal; and, if
necessary, the median lethal dose (LD50)—the
dose that kills approximately 50% of the animals. Presently, the LD50
is estimated from the smallest number of animals possible. These doses are used
to calculate the initial dose to be tried in humans, usually taken as one
hundredth to one tenth of the no-effect dose in animals.
It
is important to recognize the limitations of preclinical test-ing. These
include the following:
1.
Toxicity testing is time-consuming and expensive. Two to 6 years may be
required to collect and analyze data on toxicity before the drug can be
considered ready for testing in humans.
2.
Large numbers of animals may be needed to obtain valid pre-clinical data.
Scientists are properly concerned about this situ-ation, and progress has been
made toward reducing the numbers required while still obtaining valid data.
Cell and tis-sue culture in vitro methods and computer modeling are
increasingly being used, but their predictive value is still lim-ited. Nevertheless,
some segments of the public attempt to halt all animal testing in the unfounded
belief that it has become unnecessary.
3.
Extrapolations of therapeutic index and toxicity data from animals to humans
are reasonably predictive for many but not for all toxicities. Seeking an
improved process, a Predictive Safety Testing Consortium of five of America’s
largest pharma-ceutical companies with an advisory role by the Food and Drug
Administration (FDA) has been formed to share internally developed laboratory methods
to predict the safety of new treatments before they are tested in humans. In
2007, this group presented to the FDA a list of biomarkers for early kid-ney
damage.
4.
For statistical reasons, rare adverse effects are unlikely to be detected in
preclinical testing.
Related Topics