Chapter: Biotechnology: Protein Structure And Engineering
Designing Proteins (Protein Engineering)
Designing Proteins (Protein Engineering)
Considerable interest exists in the biotechnology industry for the engineering of proteins with increased stability when exposed to harsh conditions like elevated temperature, organic solvents and reactive chemicals, often encountered in the industrial processes. Besides, it is of great interest to explore biological adaptations to environmental stresses such as high salinity, drought, cold etc. Therefore, in order to stabilise your favourite protein, it is essential to know the cause of inactivation.
Stability in a folded protein is a balance between the stabilising (mainly hydrophobic) interactions and the tendency towards destabilisation caused by the loss of conformational entropy as the protein adopts the unfolded form. The stability of a protein may however be changed by substituting amino acids that either favour stabilising interactions in a folded protein or destabilising interactions in an inactive protein. Numerous attempts have been made on different proteins and enzymes in order to improve their properties for thermal and pH stability, solvent tolerance and solubility, catalytic potency etc. Given below is an example which has been successfully used in the detergent industry.
Improving laundry detergent Subtilisin
Subtilisin (27 kD) is a protease produced by bacteria that can digest a broad range of proteins that commonly soil clothing, see Fig. 12. The enzymatic activity of subtilisin is contributed by a catalytic triad, i.e., Ser221, His64 and Asp32 similar to chymotrypsin. Replacement of all three residues with alanine either singly or in combination results in significant loss of activity. Subtilisin represents the largest industrial market for any enzyme. To improve the efficiency of laundry detergents, detergent manufacturers supplement subtilisin in their products with various catchy slogans on the detergent box such as "stain cutter" or "biologically active enzymes".
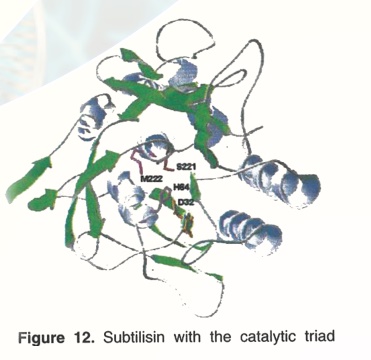
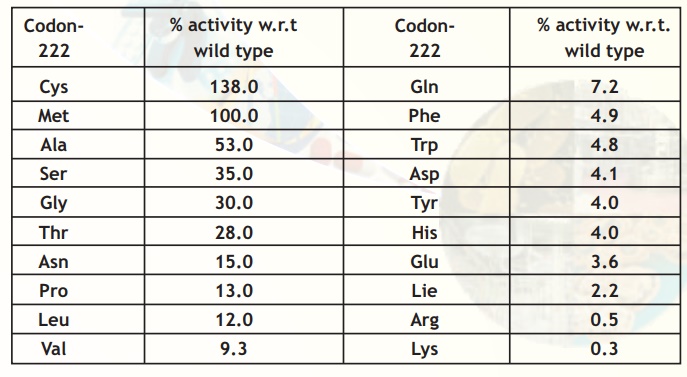
The native enzyme subtilisin is easily inactivated by bleach (up to 90%). Careful studies showed that this inactivation was due to oxidation of the amino acid residue Methionine222 in the protein molecule (Fig.12). Using site-directed mutagenesis of the subtilisin gene in E.coli, this methionine was substituted by a variety of other amino acids and the enzyme activity measured in the presence of bleach (Table 5). It was observed that substitution of Met222 with Ala222 was the best in terms of activity and stability. Nowadays, many laundry detergents contain cloned, genetically engineered or recombinant subtilisin.
Table 5. Site-directed mutagenesis at codon position 222.
Creation of Novel Proteins
Conventional vaccines have utilised heat inactivated bacteria/viruses or their surface proteins to generate immunity against various specific diseases. Often it has been observed that some of the components of the vaccines have undesirable effects such as fever and rarely one has also heard of some of the components like virus actually causing the disease due to incomplete inactivation. Since proteins are the main molecules which provide the stimulus for immunity, attempts have been made to engineer proteins having minimum deleterious effects. The specific sequences of amino acids in the protein which stimulate immune response are known as epitopes. A recombinant vaccine based on selected epitopes may provide optimal design, scope for micromanipulation, unhindered supply and safety needed for an effective vaccine. Working on these lines, a novel synthetic gene has been assembled as a first step towards developing a subunit vaccine against Hepatitis B virus.
Improving nutritional value of cereals and legumes
The cereal grains and seeds of legumes constitute a major chunk of dietary protein requirement. The seed storage proteins are synthesised and accumulated throughout seed development to serve as source of amino acid reserves at the time of seed germination. High levels of such proteins in seeds would provide an enriched amino acid source for human consumption. However deficiencies in seeds of certain essential amino acids render the cereal grains or legumes unsuitable for a balanced diet. Supplementation of diet with essential amino acids from other sources therefore becomes essential. Fig.13 gives the essential amino acid content of various cereals and commonly eaten food proteins. Essential amino acids are those which have to be obtained from food and cannot be made in our cells. From the data in the figure it is apparent that whey protein is superior to other sources especially with regard to branched amino acids- ile, leu, val, lys and trp. The branched chain amino acids (BCAA) are essential for the biosynthesis of muscle proteins. They help in increasing the bio-availability of high complex carbohydrates intake and are absorbed by muscle cells for anabolic muscle building activity. One of the theories is that during exercise the BCAAs are released from the skeletal muscle; the carbon skeleton part is used as fuel and the nitrogen part is used to make alanine which then goes to the liver where it is turned into glucose for energy. So for athletes who want to protect their existing mass, the idea is to take BCAA enriched foods before and after excercise. BCAAs reduce muscle breakdown and act as an energy source before and after exercise. Hence while maintaining exercise performance and delaying exhaustion BCAAs are very important for muscle growth. Nowadays an entire new area of sports medicine and nutrition prepare and recommend special nutrient drinks etc. which incorporate these principles.
Biological value (BV) measures the amount of protein nitrogen that is retained by the body from a given amount of protein nitrogen that has been consumed. It has been observed that the BV of whey proteins is the highest compared to rice, wheat, soya and egg proteins. Another index of protein value is the protein efficiency ratio (PER). PER is used as a measure of growth expressed in terms of weight gain of an adult by consuming 1g of food protein. The PER value of the following proteins are arranged in decreasing order- whey, milk, casein, soya, rice, wheat. The modern day approach for overcoming the nutritional deficiencies of seeds would be to engineer genes that would encode storage proteins with more of the nutritionally desirable amino acids either by inserting additional amino acids or substituting existing amino acids with new ones. Attempts are already being made on zein storage protein genes of maize to enhance its nutritional value. Introduction of entirely novel proteins that are highly enriched in specific amino acids is also being considered.
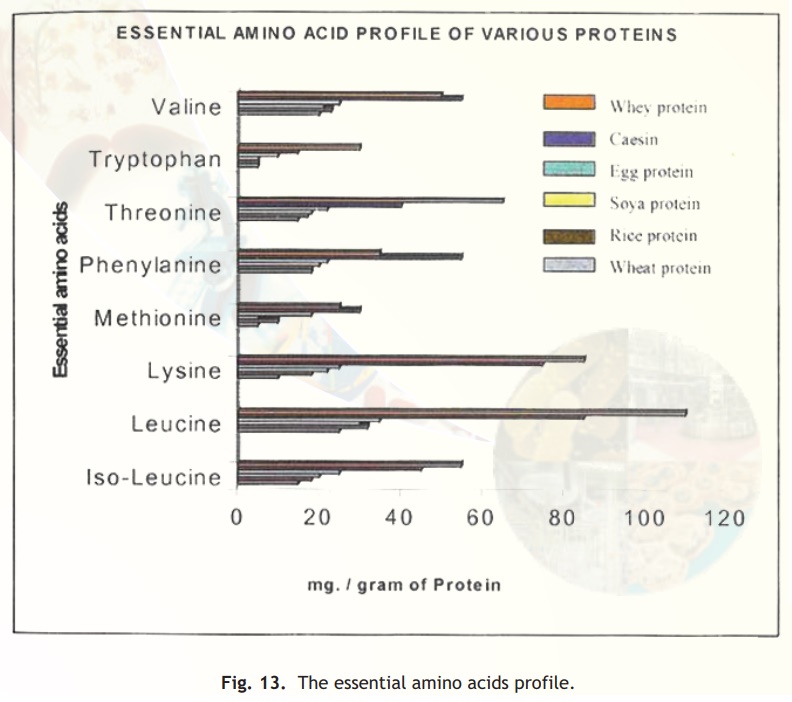
Fig. 13. The essential amino acids profile
Related Topics