Chapter: Biotechnology: Protein Structure And Engineering
Chymotrypsin, a proteolytic enzyme

Chymotrypsin, a proteolytic enzyme
As injested food makes way into the duodenum from the stomach, the proteins encounter a fierce proteolytic duotrypsin and chymotrypsin which precisely cut the linear chains into short peptides which later on are acted upon by peptidases to release amino acids. Chymotrypsin, which hydrolyses peptide bonds following bulky aromatic amino acid residues in polypeptides is actually synthesised in the pancreas and through the pancreatic duct released into the duodenum. Have you wondered why this enzyme being a powerful proteolytic enzyme does not end up cutting cellular proteins within the pancreas itself? Nature has ensured that chymotrypsin and other proteolytic enzymes are synthesised as inactive harmless precursors known as zymogens which are then activated when required only in the duodenum, their site of activity, a process called in-situ activation. This activation in molecular terms results in an alteration in its shape so that it may now be able to interact with its substrate. The inactive precursor enzyme is termed chymotrypsinogen and the fully active enzyme is called chymotrypsin. The enzyme chymotrypsin is made up of a linear chain of 245 amino acids interrupted into three peptides-A,B,C. The protein folds into a globular structure. In the 3-D structure of the enzyme three important amino acid residues, his57, asp102 and ser195 come close together in space (Fig. 4) which allows a "charge relay system" to operate as indicated in Fig 5. The negatively charged asp102 is able to hydrogen bond with the adjacent his57 partially borrowing the hydrogen ion from the latter. The his57 makes good its partial hydrogen ion loss to aspartate by attracting a hydrogen ion from the adjacent ser195 through the his57 residue much like a relay race where the baton is passed from one member to another, the difference here being that the baton is a charge.
Normally the hydroxyl group of a serine residue is not acidic (pKa 12) and this is true for all other serine residues of chymotrypsin; only ser195 becomes acidic due to the unique constellation of the three amino acid residues because the protein has folded uniquely in space. You may be curious about the importance about an acidic serine residue. The negatively charged oxygen anion is able to make a nucleophilic attack on the carbonyl carbon of the peptide bond of its substrate, loosening it so that a water molecule can hydrolyse the bond (Fig. 5). The specific site of chymotrypsin (recall that the enzyme is specific to aromatic residues) is a large space created within the enzyme active site and lined by hydrophobic residues which therefore only allow bulky aromatic, hydrophobic amino acids to bind. This binding brings the susceptible peptide bond close to the attacking ser195 residue. In chymotrypsinogen, the substrate binding site is blocked and hence the enzyme is inactive. In-situ activation of trypsin involves a proteolytic cut in chymotry p s i n o g e n which results in a conformational change, exposing the substrate binding pocket.
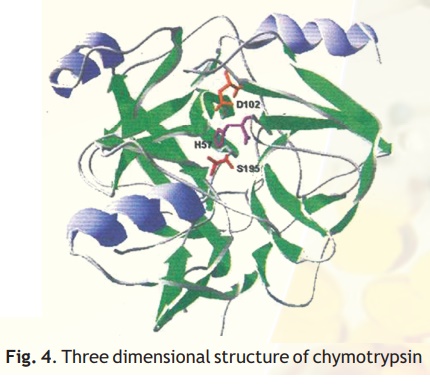
Fig. 4. Three dimensional structure of chymotrypsin
The interesting thing is that when nature has found a useful folding pattern which can cause hydrolysis of protein substrates, it repeats this in a variety of other enzymes. Trypsin, subtilisin (a proteolytic enzyme found in B. subtilis, a bacterium), thrombin (a proteolytic blood clotting factor) and the brain enzyme, acetyl choline esterase all have a reactive serine residue which is central to the catalytic mechanism.
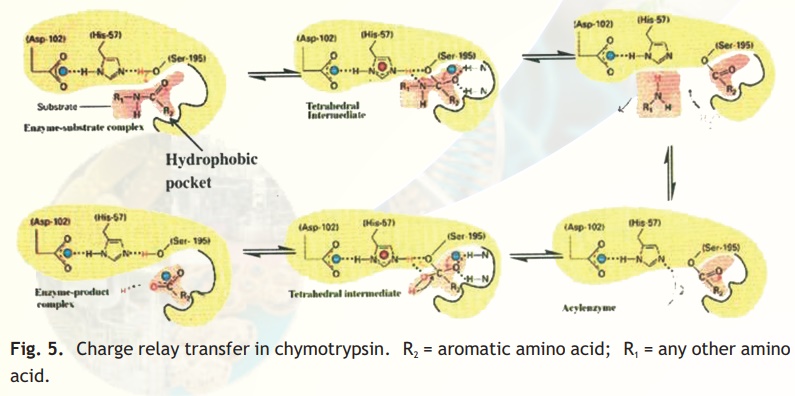
Fig. 5. Charge relay transfer in chymotrypsin. R2 = aromatic amino acid; R1 = any other amino acid.
Certain organophosphate compounds can selectively react with an acidic serine residue thereby knocking off enzyme activity. Nerve gas which was unfortunately used in the first world war had volatile serine alkylating compounds which inactivates the brain enzyme acetyl choline esterase leading to death. Nowadays derivatives of organophosphates such as malathion and parathion which are not toxic to humans are used as mosquito repellants (Mortein, Good Knight) by effecting nerve transmission in insects.
Related Topics