Chapter: Biotechnology: Protein Structure And Engineering
2-D Gel Electrophoresis
2-D Gel Electrophoresis
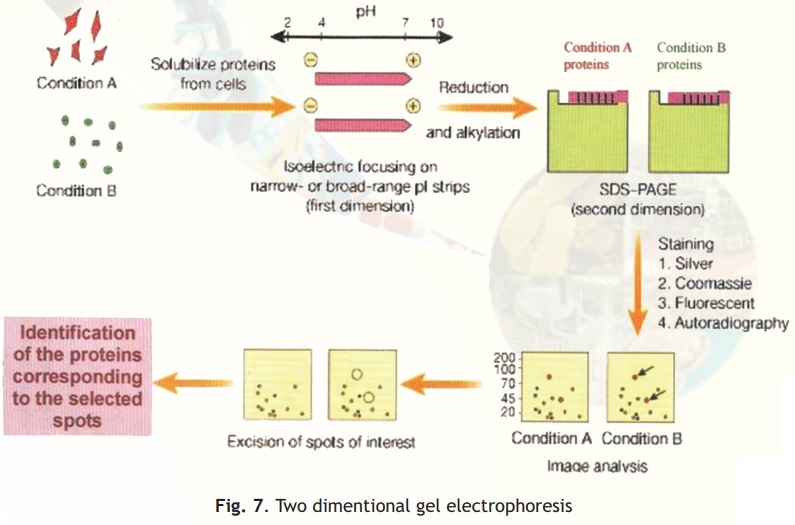
Two different techniques are combined in this procedure- Isoelectro focussing (IEF) and SDS-PAGE (Fig. 7).
In simple electrophoresis, the mobility of proteins is due to their charge, which is pH dependant. At its isoelectric pH (pI), a protein does not possess any charge and thus will not move in an applied electric field. This feature is exploited in the technique of IEF, which separates proteins on the basis of their different pI values. Usually IEF is performed in thin tube gels. A pH gradient is set up within the IEF gel by the inclusion of polymeric buffers known as ampholytes. These, like proteins have many positive and negative charges and hence varying pIs. Because of their smaller sizes they move rapidly in an electrophoretic run setting up pH gradients when they come to rest at specific distances from the anode/cathode when they have no net charge. A protein sample from a cell or any other source is then electrophoresed within these tubes wherein the different proteins separate and migrate to their pI zones. The tubes containing the separated proteins is then laid on a SDS-PAGE slab gel and electrophoresis continued at right angles to the IEF direction.
In SDS-PAGE proteins separate on the basis of their size and hence at the end of this electrophoretic run proteins are separated into 2-D patterns with high resolution as two properties of the proteins have been exploited in their separation- charge and size. Proteins in the gels are stained with silver stains or other highly sensitive dyes and can be scanned, and pictures stored into computer databases for analysis.
Related Topics