Chapter: Essentials of Psychiatry: Delirium and Dementia
Dementia of the Alzheimer Type
Degenerative Causes of Dementia
Dementia of the Alzheimer Type
Historical Perspective
In 1906 Alois Alzheimer reported a case of
presenile dementia in a 51-year-old woman who displayed progressive memory loss
and disorientation.
Two years earlier, Alzheimer had written of miliary
plaque formations that often appeared in the brains of patients with se-nile
dementia. He and his coworkers subsequently described neurofibrillary changes
and granulovacuolar degeneration in se-nile and presenile dementia (Bick,
1994). Almost 90 years later, Alzheimer’s disease is the most common form of
dementia and remains a major focus of scientific investigation.
Epidemiology
Alzheimer’s disease is the most common cause of dementia, ac-counting for 55 to 65% of all cases. There were fewer than 3 mil-lion cases diagnosed in the USA in 1980, but the Census Bureau predicted that there will be more than 10 million American citi-zens with Alzheimer’s disease by the year 2050. Prevalence of the disease doubles with every 5 years between the ages of 65 and 85 years. Onset of symptoms occurs after the age of 40 years in 96% of cases.
Some authors separate Alzheimer’s disease into
senile and presenile forms, but the two disorders represent the same
patho-logical process. Significantly, however, early-onset (that is, onset
before the age of 65) Alzheimer’s disease is associated with a more rapid
course than later-onset disease.
Alzheimer’s disease affects women three times as
often as men, for unknown reasons. Furthermore, at least one study suggests
that dementia, including Alzheimer’s, is more com-mon in black than in white
American women (Heyman et al., 1991).
Comparison of population studies in diverse countries shows strikingly similar
prevalence rates. Longitudinal studies have revealed the importance of family
history as a risk factor; however, no consistent genetic pattern has been
established. For Alzheimer’s alone, the probability of developing dementia if a
first-degree relative (parent or sibling) is afflicted is four times greater
than that of the general population, and if two or more first-degree relatives
have the disease the risk is increased eightfold compared with a normal sample
of US citizens. Among monozygotic twins 43% are concordant for the disorder,
com-pared with only 8% of dizygotic twins.
In addition to age, gender and family history, the
presence of Down syndrome, a history of head trauma and a low level of
education have been proposed as risk factors. Most studies con-cur that
individuals with trisomy 21 develop the features of AD by age 35 years;
however, studies have looked at the possibil-ity that families with a member
who has AD are more likely to produce offspring with Down syndrome and have had
inconclu-sive results. Significant head injury, as either a single incident or
a chronic occurrence as in sports injuries, increases the risk of developing
Alzheimer’s by a factor of 2. An uneducated person older than 75 years is about
twice as likely to develop dementia as one who has 8 years or more schooling,
leading to the speculation that the cognitive processes involved in obtaining
an education may be partially protective. Risk factors found in some but not
all studies include myocardial ischemia in the elderly, having a child at 40
years or older, and exposure to aluminum (Katzman and Kawas, 1994).
Pathology
The etiology and pathogenesis of Alzheimer’s
disease are un-known. Multiple agents and pathways are probably involved in
this disorder. Many hypotheses have been proposed regard-ing the cause and
progression of Alzheimer’s disease includ-ing genetic factors, slow or
unconventional viruses, defective membrane metabolism, endogenous toxins, autoimmune
disor-ders and neurotoxicity of such trace elements as aluminum and mercury.
The brains of patients with Alzheimer’s disease
contain many senile plaques, neurofibrillary tangles and Hirano’s bodies
(Figures 32.3 and 32.4). There is degeneration of nerve cells, but the
significant atrophy seen on neurodiagnostic examination may be more the result
of shrinkage of neurons and loss of dendritic spines than of actual neuronal
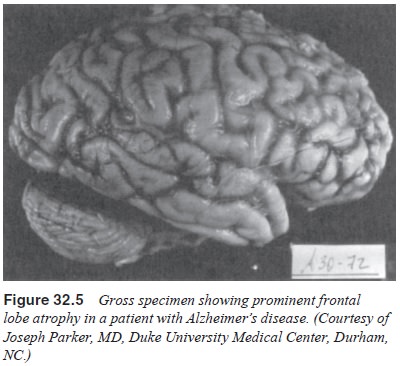
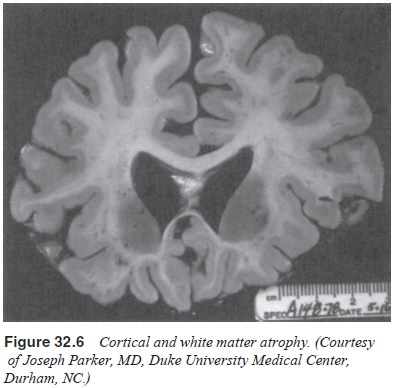
loss (Wolf, 1980). The atrophy is most apparent in the associational cortex
areas, and early decay on the primary motor and sensory areas are relatively
spared (Figures 32.5 and 32.6). Significant degenerative changes in neurons are
seen in the hippocampus, locus ceruleus and nucleus basalis of Mynert. With
advancing disease, these changes, in effect, separate the hippocampus from the
remainder of the brain. Initially, the parietal and temporal regions are most
affected by plaques and tangles, accounting for the memory impairment and
parietal lobe–associated syndromes (some apraxias, hemi-attention, anosognosia,
Gerstmann’s syndrome) occasionally associated with Alzheimer’s disease.
Neurofibrillary tangles do not correlate with the severity of
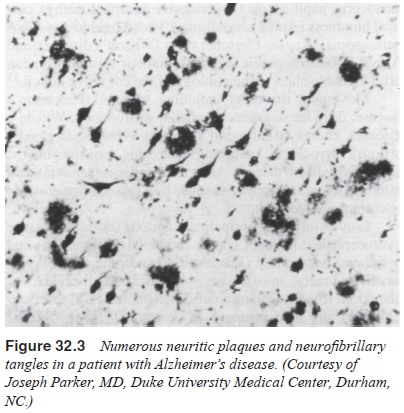
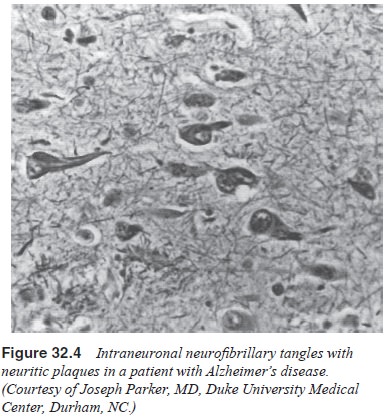
the dementia; however, the concentration of
neuritic plaques is directly associated with the severity of the disease
(Kaufman, 1990a).
Neurochemically, the brains of patients with Alzheimer’s disease exhibit significant cholinergic abnormalities (Kaufman, 1990b). There is a profound decrease in acetylcholine (ACh) in almost all patients, as well as decreased immunological activity of somatostatin- and corticotropin-releasing factors (Kaufman, 1990b). The enzyme required for ACh synthesis, choline acetyltransferase, is also greatly reduced. Other studies sug-gest involvement of noradrenergic and serotonergic systems in later-onset disease and diminished gamma-aminobutyric acid (GABA) (Kaufman, 1990b). Specifically, the noradrenergic de-ficiencies seen in younger patients may be connected to changes in the locus coeruleus, and abnormalities of serotonin to effects on the raphe nuclei (Korvath et al., 1989). The serotoninergic neurons of the raphe nuclei in patients with Alzheimer’s disease contain six to 39 times as many neurofibrillary tangles as those of age-appropriate control subjects, and noradrenergic neurons from the locus ceruleus of patients with Alzheimer’s disease show neuronal loss of 40 to 80%. Unfortunately, despite these observed neurochemical abnormalities, neurotransmitter-related treatment with cholinergic and GABAergic agents has proved largely unsuccessful.
Although the involvement of cholinergic
transmission along the hippocampus and nucleus basalis is essential to the
ability to learn new information, it seems that many of the symptoms of
Alzheimer’s disease are not explainable solely on the basis of cholinergic
abnormalities. Thus, investigators have examined a number of other potential
etiological or con-tributory agents. Some researchers have investigated the
role of beta-amyloid protein in Alzheimer’s disease, and some as-sert that this
material, a significant component of all plaques, is a major contributor to the
neurodegenerative changes in the disease as both an initiator and a promotor of
the disease. Supporting this assertion are genetic studies of families with
inheritable forms of presenile dementia, which show that dis-ease occurrence is
linked to mutations involving beta-amyloid-related systems (Kidd, 1963). This
hypothesis targets the pro-tein found in senile plaques; other investigators have
focused on the neurofibrillary tangles and the identification of a major
component of its helical filament, the tau protein. Specifically, these
researchers analyzed the possibility that modification of tau protein,
predominantly by phosphorylation, is an important feature of AD.
Aluminum, the third most common element in the
uni-verse, is absorbed from the gastrointestinal tract, lungs, na-sal passage
and skin. Crapper and Dalton (1972) reported in-creased aluminum in the brain
of patients with Alzheimer’s disease, with about a quarter of such samples
showing con-centrations three standard deviations above the control val-ues.
Other studies of bulk brain aluminum in patients with Alzheimer’s disease have
shown no such elevation. The cur-rent consensus appears to be that although
aluminum and other elements such as iron and mercury might accelerate neuronal
degeneration in AD, these elements are not primary etiologi-cal agents. The
role of genetic factors in the development of AD has received increased attention
as the role of the apoli-poprotein (APO) E4 allele as a major genetic
susceptibility risk factor has been confirmed by numerous studies (Katzman,
1994). Corder and colleagues (1993) studied 234 members of 42 families with
late-onset AD. Of 95 affected members, 80% had the E4 allele, compared with 26%
in the general popula-tion (Corder et al.,
1993). Furthermore, in these families, 91% of those homozygous for E4 had
developed Alzheimer’s dis-ease by 80 years of age – evidence that the APO E E4
allele is causing these familial cases (Corder et al., 1993). In a study of 176 autopsy specimens of confirmed AD,
Schmechel and coworkers in 1993 found that 65% of patients carried at least one
APO E E4 gene. Examination of all such studies indicates that between 25 and
40% of AD cases can be attributable to this marker, making its presence one of
the most common risk factors yet discovered for AD.
Finally, several studies suggest that changes in
membrane function, metabolism and morphology are involved in the pathol-ogy of
AD. Nonetheless, the basic molecular defect responsible for AD dementia has not
been defined.
The neuropathology of Alzheimer’s disease should be
compared with the normal neuropathic effects of aging. These include the
following:
· The leptomeninges
become more fibrotic and are more adher-ent to the brain surface with increased
opacity.
· The
ventricles show slight to moderate enlargement that in-creases with the passage
of time.
· The
distance between the dura and the brain is increased.
· Sulci
widen and gyri become narrower.
· The
number of neurons decreases slightly.
· The
weight of the brain decreases in the fourth and fifth dec-ades, with
significant decrease by the age of 80 years.
· Neurofibrillary
tangles and senile plaques occur in virtually every elderly individual by the
10th decade of life (Berg et al.,
1994).
Laboratory and Radiological Findings
The role of laboratory determinations in the
evaluation for AD is to exclude other causes of dementia, especially those that
may prove reversible or arrestable. Before death, AD is largely a diag-nosis of
exclusion. Throughout the course of this disorder, labo-ratory values are
essentially normal. Some nonspecific changes may occur, but
electroencephalography and lumbar puncture are not diagnostic. As the disease
progresses, computed tomography (CT) and magnetic resonance imaging (MRI) may
show atrophy in the cerebral cortex and hydrocephalus ex vacuo MRI may show nonspecific alteration of white matter
(leukoariosis), and eventually the electroencephalogram (EEG) shows diffuse
back-ground slowing.
Pneumoencephalography has demonstrated enlarged
ven-tricles and widening of cortical sulci in Alzheimer’s disease, and positron
emission tomography in the later stages shows de-creased cerebral oxygen and
glucose metabolism in the frontal lobes. At present, in the work-up of a
patient with a slowly pro-gressive dementia, a good family history, physical
examination, and laboratory and radiographic tests to rule out other causes of
dementia, are the most effective tools in the diagnosis of Alzhe-imer’s
disease.
Clinical Features
The course and clinical features of AD parallel
those discussed for dementia in general. Typically, the early course of AD is
difficult to ascertain because the patient is usually an unreliable inform-ant,
and the early signs may be so subtle as to go unnoticed even by the patient’s
closest associates. These early features include impaired memory, difficulty
with problem solving, preoccupa-tion with long past events, decreased
spontaneity, and an inability to respond to the environment with the patient’s
usual speed and accuracy. Patients may forget names, misplace household items,
or forget what they were about to do. Often the individuals have insight into
these memory deficits and occasionally convey their concerns to family members.
Such responses as “You’re just get-ting older”, and “I do that sometimes
myself” are common from these family members and as a result the patient
becomes de-pressed, which can further affect cognitive functioning. Anomia, or
difficulty with word finding, is common in this middle stage of Alzheimer’s
disease. Eventually the patient develops schemes, word associations and excuses
(“I never was very good in math”) to assist in retention and cover up deficits.
The patient may also employ family members as a surrogate memory (Karp, 1984).
Because memory loss is usually most obvious for
newly acquired material, the patient tries to avoid unfamiliar activities.
Typically, the patient is seen by the physician when confusion, aggression,
wandering, or some other socially undesirable be-havior ensues. At that time,
disorders of perception and language may appear. The patient often turns to a
spouse to answer ques-tions posed during the history taking. By this time the
affected individual has lost insight into his or her dementia and abandons
attempts to compensate for memory loss. Finally, in the late stage of
Alzheimer’s disease, physical and cognitive effects are marked. Disorders of
gait, extremity paresis and paralysis, seizures, peripheral neuropathy,
extrapyramidal signs and urinary incon-tinence are seen, and the patient is
often no longer ambulatory. The aimless wandering of the middle stage has been
replaced by a mute, bedridden state and decorticate posture. Myoclonus
occasionally occurs. Significantly, affective disturbances remain a distinct
possibility throughout the course of the illness. AD progresses at a slow pace
for 8 to 10 years to a state of complete helplessness.
Treatment
The two principles of management in AD are to treat
what is treatable without aggravating existing symptoms and to sup-port
caregivers, who are also victims of this disease. Despite the significant
decrease in ACh and choline acetyltransferase in Alzheimer’s disease, treatments
based on the cholinergic hypothesis have been unsuccessful (Kaufman, 1990a)
Because vasopressin levels are slightly decreased in the hippocampus of
patients with Alzheimer’s disease and somatostatin is adversely affected as
well, attempts were made to replace these agents with little effect. In the
belief that improving blood flow might be of benefit, such agents as the
metabolic enhancer and vasodilator ergoloid mesylates (Hydergine) (an ergot
alkaloid) were tried. Hydergine did seem to have some benefit; however, these
effects may have been related to its mild antidepressant action. Onset of
action of any beneficial effects of Hydergine was quite long. Corticotropin
release is promoted by corticotropin-releasing fac-tor, which is decreased in patients
with AD, but clinical trials with corticotropin were disappointing. Despite
lackluster effects of physostigmine, a second cholinesterase inhibitor has
shown promise. Tetrahydroaminoacridine (tacrine) produced significant cognitive
improvement in 16 of 17 patients with AD in an early study (Summers et al., 1986). Subsequent studies have
been less impressive, but significant improvement in a number of scales
measuring cognitive performance illustrated the benefit of this agent for some
patients. Side effects, particularly hepatic and cholinergic, were noted;
however, in 1993 the US Food and Drug Administration (USFDA) approved of
tacrine for the treatment of AD. Donepezil, an inhibitor of
acetylcholinesterase, has also been utilized in an attempt to enhance
cholinergic function by inhibiting its breakdown. This agent must be given
early in the course of the dementia.
Whereas much attention has been focused on research
aimed at understanding and altering the pathogenesis of AD, less work has been
done regarding appropriate pharmacotherapy of the varied psychological
manifestations of the disease. Depres-sion is often associated with AD. If
antidepressant medication is to be used, low doses (about one-third to one-half
of the usual ini-tial dose) are advised and only agents with minimal
anticholin-ergic activity should be employed. Appropriate choices would be the
selective serotonin reuptake inhibitors such as paroxetine, fluoxetine,
sertraline and citalopram. Sertraline and citalopram are least likely to cause
drug–drug interactions. Even these agents have the potential to increase
confusion in Alzheimer’s patients. Agents such as trazodone and mirtazapine
have occasionally been employed because of their sedating properties. If
tricyclic antidepressants are used, the secondary amines (desipramine,
nortriptyline) are recommended over the tertiary ones (am-itriptyline,
doxepin). Careful attention to the possible side effects of these agents,
particularly orthostatic hypotension, lowering of the seizure threshold,
excessive fatigue, urinary retention, con-stipation, confusion and accelerated
memory impairment, is sug-gested. Most clinicians now feel that tricyclic
antidepressants are inappropriate for this patient population.
Anxiety and psychosis, particularly paranoid
delusions, are common in AD. Benzodiazepines can be disinhibiting in such
patients and may exacerbate confusion. They should be avoided if possible. If
minor tranquilizers are required, agents with a shorter duration of action
(e.g., lorazepam, oxazepam) are preferred. Antipsychotic medications with high
anticholinergic potential (thioridazine, chlorpromazine) may also affect memory
adversely. While these agents have been favored in the past be-cause of their
tendency to produce sedation, newer agents such as olanzepine, risperdone,
quetiapine and ziprasidone, have been reported to have lower incidences of
neuroleptic-related side effects. Haloperidol has less anticholinergic activity
but has a greater tendency toward extrapyramidal effects. These agents will be
discussed in more detail in the consideration of manage-ment of delirious
states. In summary, the psychopharmacological management of AD is designed to
ameliorate cognitive deficits, if possible, control agitated, psychotic and
dangerous behavior, and treat any underlying psychiatric disorder (e.g., major
depressive disorder) that might be comorbid with dementia. The appropri-ate
management of AD entails more than psychopharmacologi-cal intervention. Other
elements of the treatment plan should be environmental manipulation and support
for the family.
In the attempt to maintain patients with
Alzheimer’s dis-ease in their homes for as long as possible, some adjustments
of their environment are important. Written daily reminders can be helpful in
the performance of daily activities. Prominent clocks, calendars and windows
are important. An effort should be made to minimize changes in the patient’s
daily activities and environment. Repeated demonstrations of how to lock
doorand windows and operate appliances are helpful, and arranging for rapid
dialing of essential telephone numbers can be useful. Maintaining adequate
hydration, nutrition, exercise and clean-liness is essential. The family of the
patient with Alzheimer’s disease is also a victim of the disease. Family
members must watch the gradual deterioration of the patient and accept that a
significant part of their own lives must be devoted to the care of the
individual. Difficult decisions about institutionalization and termination of
life support are distinct possibilities, and the pa-tients often turn their
anger and paranoia toward the caregiver. Education is a valuable treatment tool
for families. Information about the disease and peer support are available
through Alzhe-imer’s associations, and many such agencies provide family
members with a companion for the patient to allow the family some time away.
(The National Alzheimer’s Education and Re-ferral Service can be accessed by
calling 1-800-621-0379.) Many studies suggest that the primary reason for
institutionalization of these patients is the tremendous burden of care they
pose for their families. Aimless wandering seems to be a particularly
dis-turbing behavior. Unfortunately, the unfamiliar surroundings of a nursing
home often increase the patient’s level of confusion and anxiety. For these
reasons, family members are at risk for depression, anxiety disorders, insomnia
and a variety of other psychological manifestations. Should these occur, they
should be promptly treated.
Related Topics