Chapter: Basic & Clinical Pharmacology : Diuretic Agents
Collecting Tubule System - Renal Tubule Transport Mechanisms
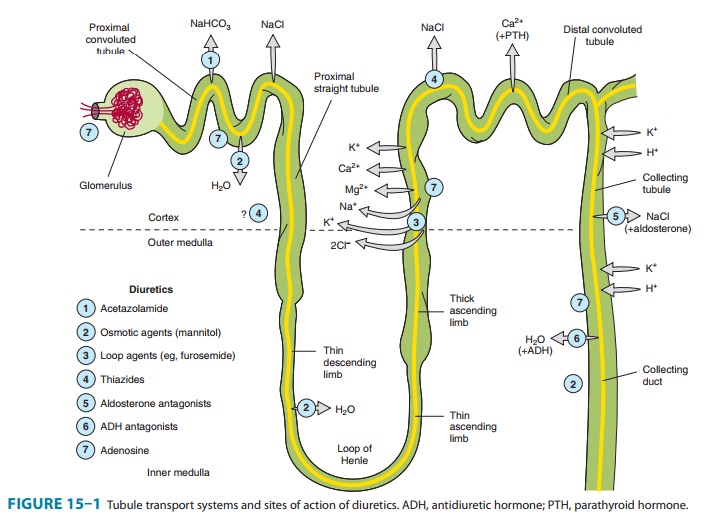
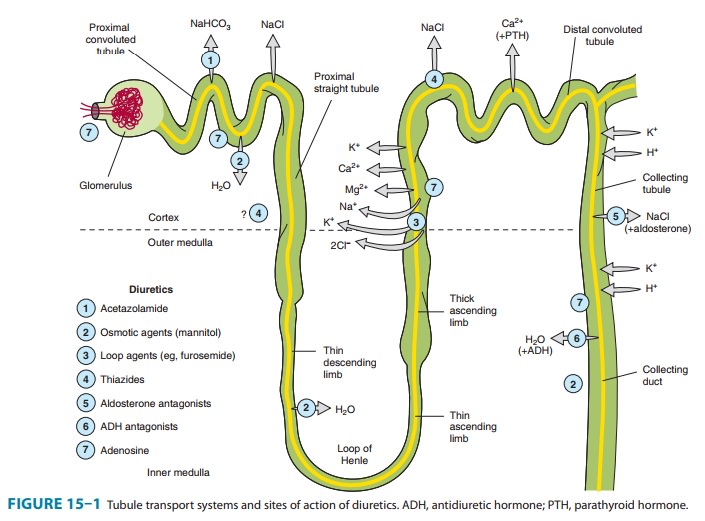
COLLECTING TUBULE SYSTEM
The
collecting tubule system that connects the DCT to the renal pelvis and the
ureter consists of several sequential tubular segments: the connecting tubule,
the collecting tubule, and the collecting duct (formed by the connection of two
or more collecting tubules). Although these tubule segments may be anatomically
distinct, the physiologic gradations are more gradual, and in terms of diuretic
activity it is easier to think of this complex as a single segment of the
nephron containing several distinct cell types. The collecting tubule system is
responsible for only 2–5% of NaCl reabsorption by the kidney. Despite this
small contribution, it plays an important role in renal physiology and in
diuretic action. As the final site of NaCl reabsorption, the collecting system
is responsible for tight regulation of body fluid volume and for determining
the final Na+ concentra-tion of the urine. Furthermore, the collecting system
is the site at which mineralocorticoids exert a significant influence. Lastly,
this is the most important site of K+ secretion by the
kidney and the site at which virtually all diuretic-induced changes in K+ balance occur.
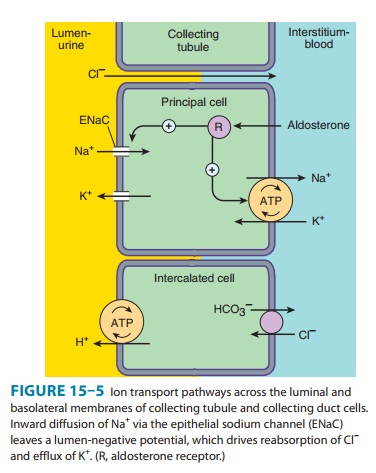
The
mechanism of NaCl reabsorption in the collecting tubule system is distinct from
the mechanisms found in other tubule seg-ments. The principal cells are the major sites of Na+, K+, and water transport
(Figures 15–5 and 15–6), and the intercalatedcells
(α,β) are the primary
sites of H+(αcells)
or bicarbonate (βcells)
secretion. The α
and β
intercalated cells are very similar, except that the membrane locations of the
H+-ATPase and Cl/HCO3−
exchanger are reversed. Principal cells do not contain apical
cotransport
systems for Na+ and other ions, unlike cells in other nephron segments.
Principal cell membranes exhibit separate ion channels for Na+ and K+. Since these channels
exclude anions, transport of Na+ or K+ leads to a net movement of charge across the membrane. Because
Na+ entry into the
principal cell pre-dominates over K+ secretion into the
lumen, a 10–50 mV lumen-negative electrical potential develops. Sodium that
enters the principal cell from the tubular fluid is then transported back to
the blood via the basolateral Na+/K+-ATPase (Figure 15–5). The 10–50 mV lumen-negative electrical
potential drives the transport of Cl− back to the blood via the
paracellular pathway and draws K+ out of cells through the apical membrane K+ channel. Thus, there
is an important relationship between Na+ delivery to the
collecting tubule system and the resulting secretion of K +. Upstream diuretics
increase Na+ delivery to this site and enhance K+ secretion. If Na+ is delivered to the
collecting system with an anion that cannot be reabsorbed as readily as Cl−
(eg, HCO3−), the lumen-negative potential is increased,
and K+ secretion is enhanced. This mechanism, combined with enhanced
aldosterone secretion due to volume depletion, is the basis for most
diuretic-induced K+ wasting. Adenosine antagonists, which act upstream at the
proximal tubule, but also at the collecting duct, are perhaps the only
diuretics that violate this principle . Reabsorption of Na+ via the epithelial Na
channel (ENaC) and its coupled secretion of K+is regulated by
aldosterone. This steroid hormone, through its actions on gene transcription,
increases the activity of both apical mem-brane channels and the basolateral Na+/K+-ATPase. This leads to
an increase in the transepithelial electrical potential and a dramatic increase
in both Na+ reabsorption and K+ secretion.
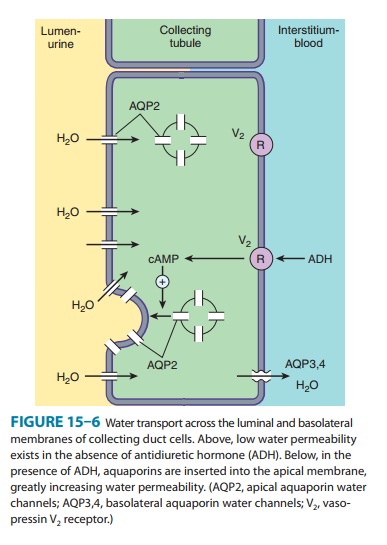
The
collecting tubule system is also the site at which the final urine
concentration is determined. In addition to their role in con-trol of Na+ absorption and K+ secretion (Figure
15–5), principal cells also contain a regulated system of water channels
(Figure 15–6). Antidiuretic hormone (ADH, also called arginine vasopres-sin,
AVP) controls the permeability of these cells to water by regulat-ing the
insertion of pre-formed water channels (aquaporin-2, AQP2) into the apical
membrane. Vasopressin receptors in the vas-culature and central nervous system
(CNS) are V1 receptors, and those in the kidney are V2
receptors. V2 receptors act via a G pro-tein-coupled, cAMP-mediated
process. In the absence of ADH, the collecting tubule (and duct) is impermeable
to water, and dilute urine is produced. ADH markedly increases water
permeability, and this leads to the formation of a more concentrated final
urine. ADH also stimulates the insertion of urea transporter UT1 molecules into
the apical membranes of collecting duct cells in the medulla.
Urea concentration in the medulla plays an important role maintaining the high osmolarity of the medulla and in the con-centration of urine. ADH secretion is regulated by serum osmola-lity and by volume status. A new class of drugs, the vaptans (see under Agents That Alter Water Excretion), are ADH antagonists.
Related Topics