Chapter: Basic & Clinical Pharmacology : Diuretic Agents
Carbonic Anhydrase Inhibitors
BASIC
PHARMACOLOGY OF DIURETIC AGENTS
CARBONIC ANHYDRASE INHIBITORS
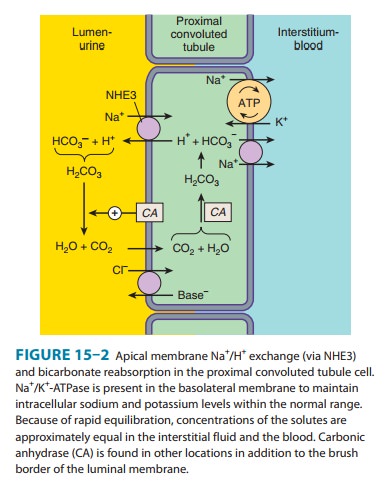
Carbonic
anhydrase is present in many nephron sites, but the predominant location of
this enzyme is the epithelial cells of the PCT (Figure 15–2), where it
catalyzes the dehydration of H2CO3 to CO2 at
the luminal membrane and rehydration of CO2 to H2CO3
in the cytoplasm as previously described. By blocking carbonic anhydrase,
inhibitors blunt NaHCO3 reabsorption and cause diuresis.
Carbonic
anhydrase inhibitors were the forerunners of modern diuretics. They were
discovered in 1937 when it was found that bacteriostatic sulfonamides caused an
alkaline diuresis and hyper-chloremic metabolic acidosis. With the development
of newer agents, carbonic anhydrase inhibitors are now rarely used as
diuretics, but they still have several specific applications that are discussed
below. The prototypical carbonic anhydrase inhibitor is acetazolamide.
Pharmacokinetics
The
carbonic anhydrase inhibitors are well absorbed after oral administration. An
increase in urine pH from the HCO3− diuresis is apparent
within 30 minutes, is maximal at 2 hours, and persists for 12 hours after a
single dose. Excretion of the drug is by secre-tion in the proximal tubule S2
segment. Therefore, dosing must be reduced in renal insufficiency.
Pharmacodynamics
Inhibition
of carbonic anhydrase activity profoundly depresses HCO3−
reabsorption in the PCT. At its maximal safe dosage, 85% of the HCO3−
reabsorptive capacity of the superficial PCT is inhibited. Some HCO3−
can still be absorbed at other nephron sites by carbonic anhydrase–independent
mechanisms, so the overall effect of maximal acetazolamide dosage is only about
45% inhibition of whole kidney HCO3− reabsorption.
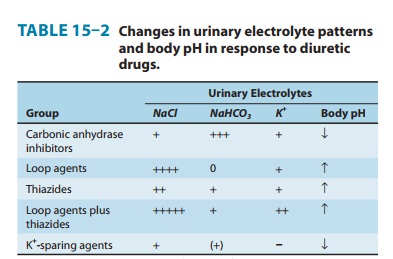
Nevertheless, carbonic anhydrase inhibition causes significant HCO3−
losses and hyperchloremic metabolic acidosis (Table 15–2). Because of reduced
HCO3− in the glomerular filtrate and the fact that HCO3−
depletion leads to enhanced NaCl reabsorption by the remainder of the nephron,
the diuretic efficacy of acetazolamide decreases significantly with use over
several days.
At
present, the major clinical applications of acetazolamide involve carbonic
anhydrase–dependent HCO3− and fluid transport at sites
other than the kidney. The ciliary body of the eye secretes HCO3−
from the blood into the aqueous humor. Likewise, forma-tion of cerebrospinal
fluid by the choroid plexus involves HCO3− secretion.
Although these processes remove HCO3− from the blood (the
direction opposite of that in the proximal tubule), they are similarly
inhibited by carbonic anhydrase inhibitors.
Clinical Indications & Dosage (Table 15–3)
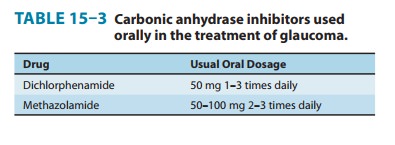
A. Glaucoma
The
reduction of aqueous humor formation by carbonic anhy-drase inhibitors
decreases the intraocular pressure. This effect is valuable in the management
of glaucoma, making it the most common indication for use of carbonic anhydrase
inhibitors. Topically active agents, which reduce intraocular pressure without
producing renal or systemic effects, are available (dorzolamide, brinzolamide).
B. Urinary Alkalinization
Uric
acid and cystine are relatively insoluble and may form stones in acidic urine.
Therefore, in cystinuria, a disorder of cystine reab-sorption, solubility of
cystine can be enhanced by increasing uri-nary pH from 7.0 to 7.5 with carbonic
anhydrase inhibitors. In the case of uric acid, pH needs to be raised only to
6.0 or 6.5. In the absence of HCO3− administration, these
effects of acetazol-amide last only 2–3 days, so prolonged therapy requires
oral HCO3−. Excessive urinary alkalinization can lead to
stone forma-tion from calcium salts , so urine pH should be fol-lowed during
treatment with acetazolamide.
C. Metabolic Alkalosis
Metabolic
alkalosis is generally treated by correction of abnor-malities in total body K+, intravascular
volume, or mineralocorti-coid levels. However, when the alkalosis is due to
excessive use of diuretics in patients with severe heart failure, replacement
of intra-vascular volume may be contraindicated. In these cases, acetazol-amide
can be useful in correcting the alkalosis as well as producing a small
additional diuresis for correction of volume overload. Acetazolamide can also
be used to rapidly correct the metabolic alkalosis that may appear following
the correction of respiratory acidosis.
D. Acute Mountain Sickness
Weakness,
dizziness, insomnia, headache, and nausea can occur in mountain travelers who
rapidly ascend above 3000 m. The symp-toms are usually mild and last for a few
days. In more serious cases, rapidly progressing pulmonary or cerebral edema
can be life-threatening. By decreasing cerebrospinal fluid formation and by
decreasing the pH of the cerebrospinal fluid and brain, acetazol-amide can
increase ventilation and diminish symptoms of moun-tain sickness. This mild
metabolic central and cerebrospinal fluid (CSF) acidosis is also useful in the
treatment of sleep apnea.
E. Other Uses
Carbonic
anhydrase inhibitors have been used as adjuvants in the treatment of epilepsy
and in some forms of hypokalemic periodic paralysis. They are also useful in
treating patients with CSF leak-age (usually caused by tumor or head trauma,
but often idio-pathic). By reducing the rate of CSF formation and intracranial
pressure, carbonic anhydrase inhibitors can significantly slow the rate of CSF
leakage. Finally, they also increase urinary phosphate excretion during severe
hyperphosphatemia.
Toxicity
A. Hyperchloremic Metabolic Acidosis
Acidosis
predictably results from chronic reduction of body HCO3−
stores by carbonic anhydrase inhibitors (Table 15–2) and limits the diuretic
efficacy of these drugs to 2 or 3 days. Unlike the diuretic effect, acidosis
persists as long as the drug is continued.
B. Renal Stones
Phosphaturia
and hypercalciuria occur during the bicarbonaturic response to inhibitors of
carbonic anhydrase. Renal excretion of solu-bilizing factors (eg, citrate) may
also decline with chronic use. Calcium salts are relatively insoluble at
alkaline pH, which means that the potential for renal stone formation from
these salts is enhanced.
C. Renal Potassium Wasting
Potassium
(K+) wasting can occur
because the increased Na+ pre-sented to the collecting tubule (with HCO3−)
is partially reab-sorbed, increasing the lumen-negative electrical potential in
that segment and enhancing K+ secretion. This effect can be counter-acted by simultaneous
administration of potassium chloride or a K+-sparing diuretic.
Potassium wasting is theoretically a problem with any diuretic that presents
increased Na+ delivery to the collect-ing tubule. However, the new adenosine
A1-receptor antagonists appear
to avoid this toxicity by blunting Na+ reabsorp-tion in the
collecting tubules as well as the proximal tubules.
D. Other Toxicities
Drowsiness
and paresthesias are common following large doses of acetazolamide. Carbonic
anhydrase inhibitors may accumulate in patients with renal failure, leading to
nervous system toxicity. Hypersensitivity reactions (fever, rashes, bone marrow
suppres-sion, and interstitial nephritis) may also occur.
Contraindications
Carbonic
anhydrase inhibitor–induced alkalinization of the urine decreases urinary
excretion of NH4+ (by converting it to rapidlyreabsorbed NH3) and may
contribute to the development of hyperammonemia
and hepatic encephalopathy in
patients withcirrhosis.
Related Topics